PROPOSED RULES
(Board of Pharmacy)
Original Notice.
Preproposal statement of inquiry was filed as WSR 06-08-104 and 06-23-079.
Title of Rule and Other Identifying Information: The board of pharmacy is proposing amendments to WAC 246-863-095 Pharmacist's professional responsibilities and new section WAC 246-869-010 Pharmacies' responsibilities, to promote patient safety and access to health care by emphasizing the professional responsibilities of pharmacists and pharmacies.
Hearing Location(s): Renton Community Center, 1715 Maple Valley Highway, Renton, WA 98057, on March 29, 2007, at 9:15 a.m.
Date of Intended Adoption: March 30, 2007.
Submit Written Comments to: Doreen E. Beebe, Department of Health, Board of Pharmacy, P.O. Box 47863, Olympia, WA 98504-7863, web site http://www3.doh.wa.gov/policyreview/, fax (360) 586-4359, by March 23, 2007.
Assistance for Persons with Disabilities: Contact Doreen E. Beebe by March 21, 2007, TTY (800) 833-6388 or 711.
Purpose of the Proposal and Its Anticipated Effects, Including Any Changes in Existing Rules: The proposed rules meet the goals and objectives of the statute by promoting patient safety and access to health care. The proposed rules provide clear expectations to assure patients have access to safe and appropriate medication therapy by eliminating barriers that would prevent patients from receiving timely access to their lawful prescribed or therapeutically equivalent drugs and devices.
Reasons Supporting Proposal: The proposed rules are needed to minimize barriers to health care and to reduce risks for patients' health where there may be an emergent need for a prescribed drug or device. Pharmacy clients should receive appropriate drugs and treatments without fear of intimidation, discrimination, or interference by pharmacy staff. Rules are needed to clarify and enforce these requirements; guidance would not have the same effect nor be enforceable.
Statutory Authority for Adoption: RCW 18.64.005, 18.130.050.
Statute Being Implemented: RCW 18.64.005, 18.130.180.
Rule is not necessitated by federal law, federal or state court decision.
Name of Proponent: Board of pharmacy, governmental.
Name of Agency Personnel Responsible for Drafting, Implementation and Enforcement: Lisa Salmi, Board of Pharmacy, P.O. Box 47863, Olympia, WA 98504-7863, (360) 236-4825.
A small business economic impact statement has been prepared under chapter 19.85 RCW.
WAC 246-863-095: The proposed rule states that it is a pharmacist's primary responsibility to ensure patients receive safe and appropriate medication therapy.
The proposed rule:
(1) Prohibits a pharmacist from delegating the decision to not dispense a lawful prescribed drug or device to pharmacy support staff.
(2) Provides grounds for discipline when a pharmacist, pharmacy intern, or pharmacy ancillary personnel engages in or permits the following conduct that is unprofessional.
(a) Destroy unfilled lawful prescription.
(b) Refuse to return unfilled lawful prescriptions.
(c) Violate a patient's privacy.
(d) Discriminate against patients or their agent in a manner prohibited by state or federal laws.
(e) Intimidate or harass a patient.
Please note that proposed amendments to WAC 246-863-095 are not analyzed within this small business economic impact statement (SBEIS) because the rule affects individual pharmacists, but does not create costs for businesses.
WAC 246-869-010: The proposed rule states that pharmacies have a duty to deliver/distribute lawful prescribed drugs and devices or provide a therapeutically equivalent drug or device to patients in a timely manner. The rule establishes requirements for a pharmacy to assure patients have access to lawfully prescribed and clinically safe medication therapy when a pharmacist cannot dispense.
The proposed rule:
(1) Provides examples of circumstances when it may be appropriate for a pharmacy not to deliver/distribute lawful prescribed drugs, devices, or provide therapeutically equivalent drugs. The list is not inclusive but validates additional circumstances as substantially similar to those listed in the proposed rule. The circumstances listed include: National or state emergencies or guidelines affect the availability, usage or supply; potentially fraudulent prescriptions; lack of specialized equipment or expertise to safely produce, store or dispense a pharmaceutical; or when a pharmacy is not compensated for its usual and customary or contracted charge.
(2) Requires pharmacies to provide patients with a timely alternative to appropriate therapy when the drug is not in stock because it is not customarily needed by the pharmacy's patients, or the drug is temporarily out-of-stock.
| Proposed Rule Options for Out-of-Stock Drugs |
||
| Contact prescriber for alternative drug therapy | Return prescription to patient | Refer patient to another pharmacy that will fill the prescription |
(a) Destroy unfilled lawful prescription.
(b) Refuse to return unfilled lawful prescriptions.
(c) Violate a patient's privacy.
(d) Discriminate against patients or their agent in a manner prohibited by state or federal laws.
(e) Intimidate or harass a patient.
2. Is an SBEIS Required for this Rule? Yes, an SBEIS is required because the proposed rule may impose more than minor costs on pharmacies. The report analyzes the probable compliance costs of the proposed rules and compares the cost of compliance for small businesses with the costs of compliance for large businesses.
3. Which Industries are Affected by this Rule? The proposed rule can be expected to affect businesses identified by the standard industrial classification code 5912 for drug stores and proprietary stores that engage in the retail sale of prescription drugs, proprietary drugs, and nonprescription medicines and may also carry a number of related lines, such as cosmetics, toiletries, tobacco, and novelty merchandise. Small pharmacy businesses are those businesses with fifty or fewer employees.
4. What are the Costs of Complying with this Rule for Small Businesses (Those with Fifty or Fewer Employees) and for the Largest 10% of Businesses Affected?
Background: The Washington board of pharmacy (board) is proposing adoption of rules amending chapter 246-863 WAC, Pharmacist's professional responsibilities and chapter 246-869 WAC, Pharmacy licensing. The SBEIS will specifically address costs issued related to the adoption of amendments to chapter 246-869 WAC. The statutes authorizing the board to adopt the proposed rule amendments are RCW 18.64.005 and 18.130.050.
The proposed amendment establishes requirements for pharmacies to deliver lawfully prescribed drugs and devices or provide a therapeutically equivalent drug to patients in a timely manner. The proposed rule will improve patient access to safe and appropriate health care by emphasizing the professional responsibilities of pharmacies.
Probable Costs of the Proposed Rules: To analyze the probable costs of the proposed rule, we must first look at the processes a pharmacy must follow in order to comply. Many of these steps are considered customary practice in a pharmacy and will not impose additional costs.
 |
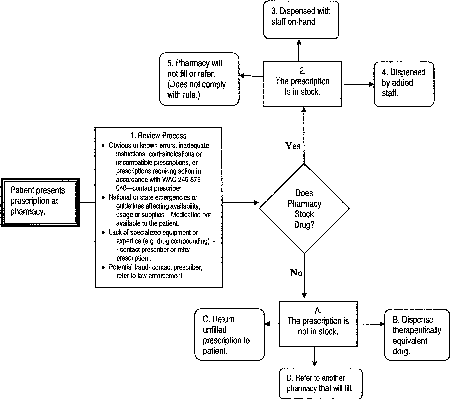
The first scenario, captured in steps 2-5, illustrates the expectations provided in subsection (1) of the proposed rule, WAC 246-869-010. Compliance cost is defined as a loss in current revenue, a loss in assets, or higher operating costs.
| • | Step 1, the pharmacist conducts a professional review of the prescription to determine the appropriateness of filling the prescription. |
| • | Step 3, after a thorough review of the prescription, the pharmacy delivers with staff on-hand. There is no compliance cost. |
| • | Step 4, after a thorough review of the prescription, the pharmacy complies with the proposed rule by having additional staff available to ensure the patient is delivered the appropriate medication therapy in situations when another pharmacist objects. The probable cost of additional staffing falls mainly to community pharmacies (small businesses) compared to corporate/chain pharmacies (large businesses) simply because of the availability of additional pharmacists. Corporate pharmacies tend to deliver/dispense larger volumes of prescriptions that support multiple pharmacists on duty at one time or overlapping shifts. Chain pharmacies often use "floaters," staff that can assist at various locations as needed. On the other hand, community pharmacies tend to have one pharmacist per shift and may have difficulty in finding part-time/on-call pharmacists. |
| • | Step 5, the pharmacy is not in compliance with the proposed rule and may be subject to disciplinary actions. |
The second scenario, captured in steps A - D, illustrates the expectations provided in subsection (3) of proposed WAC 246-869-010. In this scenario, the medication/device inventory is established in compliance with WAC 246-869-150.
| • | Step B - After a thorough review of the prescription, the pharmacist contacts the prescriber to address concerns, when appropriate. In this situation, a therapeutically equivalent product is identified and dispensed. |
| • | Step C and D - After a thorough review of the prescription, the pharmacist contacts the prescriber, when appropriate. In this situation, the pharmacy has determined that it is unable to provide a timely alternative for appropriate therapy. By request of the patient, the prescription is returned (step C) or the prescription is transferred to a pharmacy of the patient's/agent's choice that will fill the prescription in a timely manner (step D). |
| • | Returning soon-to-expire medication inventory to wholesalers/manufacturers. |
| • | More frequent pharmaceutical deliveries - up to six days a week - requiring less inventory on hand. |
| • | Pharmacies commonly borrow medications from each other when needed. |
To assist in assessing the probable costs of the adoption of the proposed rule amendments, the board surveyed a random selection of Washington pharmacies. The survey focused on current pharmacy practice and asked about the anticipated cost of complying with the proposed rule. Responses to the survey represented 39% of the one thousand three hundred seventy pharmacies located in Washington state.
Table 1
| Rural | Urban | Unknown | Retail Chain | Totals |
| 38 | 67 | 7 | 428 | 540 |
To obtain information about compliance with the rule, the board asked pharmacies specifically, "If a rule is adopted that required a pharmacy to dispense all lawful prescribed prescriptions, how might your pharmacy comply?" Although there is some resistance to the proposed rules the majority of pharmacies in each category indicated that they would comply with the rule.
Table 2
| Number of pharmacies responding | Rural (38) | Urban (67) | Pharmacy
Chains (9 corporations) |
| Number and percent of pharmacies indicated that they [will] be able to comply with the rule. | 32 (84%) | 62 (92%) | 9 (100%) |
Table 3
| Number of pharmacies responding | Rural (38) | Urban (67) | Pharmacy
Chains (9 corporations) |
| Number and percent of pharmacies that currently have procedures in place if a pharmacist refuses to dispense. | 3 (8%) | 7 (10%) | 5 (55%) |
Table 4
| Rural (38) | Urban (67) | Chain
Pharmacies
(428)
Representing 9 corporations |
|
| Pharmacies indicating additional pharmacists needed. | 1 pharmacy | 6 pharmacies | 2 corporations |
| Cost of additional pharmacists needed based on $80,000 per pharmacist. | $80,000 | $80,000 cost to most pharmacies replying* | $14,194 average cost per pharmacy** |
| Pharmacies indicating a probable cost of less than $1,000. | 1 pharmacy | 7 pharmacies | 0 corporations |
| Pharmacies indicating no additional cost. | 26 pharmacies | 50 pharmacies | 3 corporations |
| Pharmacies that did not answer the question. | 10 pharmacies | 4 pharmacies | 4 corporations |
1.5 equals $120,000.
**11 pharmacists at $80,000 are shared at 62 pharmacies: ($880,000) divided by 62 equals a $14,194 average per pharmacy.
Of the pharmacies surveyed, 87% anticipate no additional
staffing costs. For the pharmacies that employ pharmacists
that will not dispense certain drugs it is estimated that the
added yearly staffing costs will average $80,000 for small
pharmacies and $14,194 for chain store pharmacies (large
businesses).
These assumptions illustrate the likelihood that the proposed rules may impose a disproportionate financial impact on small businesses. Many health care organizations and associations have reported a serious shortage of pharmacists for a number of years. The shortage can be attributed to growth in prescription volume and new employment opportunities for pharmacists.
79% of community pharmacies responding to the survey rated the possibilities of finding an additional or temporary pharmacist as "very difficult" or "impossible." 67% of corporate retail chain pharmacies echoed these concerns.
However, as stated earlier in this analysis, the responsibilities established in the proposed rule mirror those processes that are customary practice in pharmacies today and would not result in additional costs. Additional pharmacist staffing costs and recruitment challenges pose real obstacles for any pharmacy that must institute alternatives to fulfilling its duty to deliver lawfully prescribed drugs and devices (as illustrated in step 4 in diagram 1).
Probable Benefits: The board of pharmacy has determined that the probable benefits of the proposed rules are greater than its probable costs to those that must comply. Costs of complying with the proposed rules must be balanced against the significant medical and social cost of not receiving a time-dependent medication in a timely manner. Access to medication is a critical factor in an individual's health and the efficacy of some medications is directly related to receiving the medication within a specified time.
Healthcare providers stress the importance of taking medication as prescribed. For example, when a patient has an infection they are instructed to take the entire supply of antibiotics prescribed. Compliance or adherence refers to their ability to take their medications as prescribed. People who comply have better results in combating diseases than those who do not.
For example, human immunodeficiency virus (HIV) medications are highly time sensitive. An HIV patient must regularly take the HIV drugs prescribed to suppress the virus. The consequences of missing as few as three doses can result in the virus mutating. If the virus mutates the current drug regimen is no longer effective, requiring new tests to determine what new combination of drugs may be effective. New drugs are usually less effective and more expensive. Given that the mutation is permanent, the ultimate consequence is that the patient's probability of long-term survivability can be greatly diminished.
Each time an HIV patient infection is prevented by treatment; there is a cost avoidance of $303,000a. Healthcare providers caring for HIV patients refer to the "seventy-two hour rule." When a patient misses medication doses, the drug levels fall and the virus is able to multiply. The "seventy-two hours rule" refers to the time beyond which the virus can mutate and become resistant. If the patient has a gap in taking their medication longer than seventy-two hours, the provider must repeat expensive genotype and phenotype lab tests which cost from $500-1,000 each to establish whether treatment failure has occurred.b
HIV patients who develop viral resistance have to use what is called "salvage therapy." The medications used in salvage therapy have far greater side effects and one of the drugs used must be given by injection, at a cost of $1,500 to $1,800 each month. During this time the patient has an increased ability to transmit the HIV virus to others. The patient is also immunosuppressed and vulnerable to serious infections.
Emergency contraceptive pills would be another example of a time-dependent medication. In the United States, about 50% of all pregnancies are unintended. This is the highest rate of all industrialized nations. If a woman receives emergency contraceptive pills within seventy-two hours of intercourse, there is a high probability of preventing pregnancy. Emergency contraceptive pills effectively prevent unintended pregnancy at a cost of $35-$45. If barriers impede the timely administration of this contraception, pregnancy is likely to occur. Unintended pregnancies have been shown to have significant quantitative and qualitative costs.c
When a woman has an unintended pregnancy, the impacts of an abortion may have serious emotional and health risks. The long-term costs associated with unwanted pregnancies have been calculated to be in the hundreds of thousands of dollars. The costs can be thousands of times more expensive than the cost of the emergency contraceptive pills prescription.
A new analysis by the Guttmacher Institute, published in August 2006 as part of the Institute's Occasional Report series, looked at the expanding eligibility for Medicaid-covered contraceptive services as a potential way of reducing unplanned pregnancy and, thereby, abortion. The findings suggest that providing access to contraceptive services for all Medicaid-funded women would be [a] very cost-effective program. This approach would avert $2.3 billion in costs from unplanned births and results in a net savings of $1.5 billion in Medicaid costs in the third year of the program's operation.d
A similar case can be made for other time-dependent medications or devices such as insulin and diabetic syringes, emergency contraceptive pills and erectile dysfunction medications.
5. Does the Rule Impose a Disproportionate Impact on Small Businesses? Yes, the cost of additional staffing of rule compliance for community pharmacies that reported a need to hire staff to comply with the proposed rule is much greater than the average cost of additional staffing for corporate pharmacies that indicated a need to hire staff to comply. However, only one of thirty-eight small rural pharmacies, and six of sixty-seven small urban pharmacies indicated a need to hire an additional pharmacist in order to comply with the rule. More than two thirds of small rural and urban pharmacies surveyed said they would incur no costs of complying with the proposed rule.
6. If the Rule Imposes a Disproportionate Impact on Small Businesses, What Efforts Were Taken to Reduce That Impact (Or Why Is it Not "Legal and Feasible" to Do So) By:
(a) Reducing, modifying, or eliminating substantive regulatory requirements? Reducing, modifying, or eliminating substantive regulatory requirements is not legal or feasible because the proposed rule establishes a duty to dispense prescriptions as a universal requirement to the practice of pharmacy. Prescriptions that must be taken within a short timeframe by the patient need a timely response from the pharmacy to provide the medications. On the other hand, the rule reduces the impact by including customary pharmacy practices when the time-dependent medication is not in stock.
The pharmacist may call the prescriber for a therapeutically equivalent drug, return the prescription to the patient, or refer the patient to another pharmacy with the needed medication.
Many of the responding pharmacies, who don't stock the time-sensitive medication, indicated they have or will develop a referral system as the least costly alternative.
For those pharmacies who allow the right of refusal to their pharmacists, the least cost alternative is to have pharmacist coverage with on-call pharmacist, or in the case of the larger pharmacies having at least one pharmacist on duty that will dispense the medication.
(b) Simplifying, reducing, or eliminating record-keeping and reporting requirements? The board considered simplifying, reducing, or eliminating record-keeping and reporting requirements as a means to reduce the impact of the rule on small businesses. Record-keeping requirements for pharmacies are defined in federal and state laws. The board does not have the authority to change federal or state law.
(c) Reducing the frequency of inspections? The adoption of the proposed rule does not impact the board's inspection process. Reducing inspections would not be a viable way of reducing costs to affected small businesses. Conducting inspections is necessary to protect public health and safety.
(d) Delaying compliance timetables? The board has not considered delaying implementation of the rule to give affected businesses time to find ways they can comply. The intent of the rule is to require timely compliance by pharmacies and pharmacists in delivering/dispensing appropriate medication therapy to patients. Timeliness of medication delivery to the patient is the focus of proposed rules. To delay compliance with the proposed rules would not be in the best interest of the public.
(e) Reducing or modifying fine schedules for noncompliance? Assuring timely access to medications is a critical factor in an individual's health. The efficacy of some medications is directly related to receiving the medication within a specified time. Reducing fines for small businesses who decide not to comply with the proposed rule does not meet the goal of the proposed rule.
(f) Any other mitigation techniques?
a) The rule applies to pharmacies, as well as pharmacists, to provide pharmacies with flexibility in staffing to meet the requirements of the proposed rule.
b) The rule excludes national or state emergencies affecting the availability or supplies of drugs and devices.
c) The proposed rule excludes specialized pharmacy practices that only provide limited prescriptions like compounded drugs or nuclear medicine drugs.
d) Within the one hundred twelve community pharmacies and four hundred twenty-eight corporate pharmacies surveyed, four hundred four pharmacists have a collaborative drug therapy agreement. The agreements allow the pharmacists to both prescribe and dispense medications in a timely manner, allowing even greater access to drugs or devices.
7. How are Small Businesses Involved in the Development of this Rule? Small pharmacies have been involved in the development of this rule through stakeholder meetings, letters and messages sent to the board of pharmacy, representation on the board, and participation in a pharmacy cost-of-rule survey sent to rural and urban pharmacies.
a JS Gallant. Moore News Quarterly vol 1(1) December 2000.
b Richard Aleshire, DOH HIV Program, personal communication.
c SK Henshaw. Unintended Pregnancy in the U.S. Family Planning Perspectives. 30 (Jan-Feb) 24-29, 1998.
d The Guttmacher Institute. (2006) U.S. Teenage Pregnancy Statistics National and State Trends and Trends by Race and Ethnicity. New York: The Guttmacher Institute.
A copy of the statement may be obtained by contacting Doreen E. Beebe, Board of Pharmacy, P.O. Box 47863, Olympia, WA 98504-7863, phone (360) 236-4834, fax (360) 586-4359, e-mail doreen.beebe@doh.wa.gov.
A cost-benefit analysis is required under RCW 34.05.328. A preliminary cost-benefit analysis may be obtained by contacting Doreen E. Beebe, Board of Pharmacy, P.O. Box 47863, Olympia, WA 98504-7863, phone (360) 236-4834, fax (360) 586-4359, e-mail doreen.beebe@doh.wa.gov.
February 15, 2007
Lisa Salmi
Acting Executive Director
OTS-9303.3
AMENDATORY SECTION(Amending WSR 96-02-005, filed 12/20/95,
effective 1/20/96)
WAC 246-863-095
Pharmacist's professional
responsibilities.
(1) A pharmacist's primary responsibility
is to ensure patients receive safe and appropriate medication
therapy.
(2) A pharmacist shall not delegate the following professional responsibilities:
(a) Receipt of a verbal prescription other than refill authorization from a prescriber.
(b) Consultation with the patient regarding the
prescription, both prior to and after the prescription filling
and/or regarding any information contained in a patient
medication record system provided that this shall not
((preclude a)) prohibit pharmacy ((assistant)) ancillary
personnel from providing to the patient or the patient's
health care giver certain information where no professional
judgment is required such as dates of refills or prescription
price information.
(c) Consultation with the prescriber regarding the patient and the patient's prescription.
(d) Extemporaneous compounding of the prescription
((provided that)), however, bulk compounding from a formula
and IV admixture products prepared in accordance with chapter 246-871 WAC may be performed by a ((level A)) pharmacy
((assistant)) technician when supervised by a pharmacist.
(e) Interpretation of data in a patient medication record system.
(f) Ultimate responsibility for all aspects of the completed prescription and assumption of the responsibility for the filled prescription, such as: Accuracy of drug, strength, labeling, proper container and other requirements.
(g) Dispense prescriptions to patient with proper patient information as required by WAC 246-869-220.
(h) Signing of the poison register and the Schedule V controlled substance registry book at the time of sale in accordance with RCW 69.38.030 and WAC 246-887-030 and any other item required by law, rule or regulation to be signed or initialed by a pharmacist.
(i) Professional communications with physicians, dentists, nurses and other health care practitioners.
(((2))) (j) Decision to not dispense lawfully prescribed
drugs or devices or to not distribute drugs and devices
approved by the U.S. Food and Drug Administration for
restricted distribution by pharmacies.
(3) Utilizing personnel to assist the pharmacist.
(a) The responsible pharmacist manager shall retain all professional and personal responsibility for any assisted tasks performed by personnel under his or her responsibility, as shall the pharmacy employing such personnel. The responsible pharmacist manager shall determine the extent to which personnel may be utilized to assist the pharmacist and shall assure that the pharmacist is fulfilling his or her supervisory and professional responsibilities.
(b) This does not preclude delegation to an intern or extern.
(4) It is considered unprofessional conduct for any person authorized to practice or assist in the practice of pharmacy to engage in any of the following:
(a) Destroy unfilled lawful prescription;
(b) Refuse to return unfilled lawful prescriptions;
(c) Violate a patient's privacy;
(d) Discriminate against patients or their agent in a manner prohibited by state or federal laws; and
(e) Intimidate or harass a patient.
[Statutory Authority: RCW 18.64.005. 96-02-005, § 246-863-095, filed 12/20/95, effective 1/20/96.]
OTS-9364.3
NEW SECTION
WAC 246-869-010
Pharmacies' responsibilities.
(1)
Pharmacies have a duty to deliver lawfully prescribed drugs or
devices to patients and to distribute drugs and devices
approved by the U.S. Food and Drug Administration for
restricted distribution by pharmacies, or provide a
therapeutically equivalent drug or device in a timely manner
consistent with reasonable expectations for filling the
prescription, except for the following or substantially
similar circumstances:
(a) Prescriptions containing an obvious or known error, inadequacies in the instructions, known contraindications, or incompatible prescriptions, or prescriptions requiring action in accordance with WAC 246-875-040.
(b) National or state emergencies or guidelines affecting availability, usage or supplies of drugs or devices;
(c) Lack of specialized equipment or expertise needed to safely produce, store, or dispense drugs or devices, such as certain drug compounding or storage for nuclear medicine;
(d) Potentially fraudulent prescriptions; or
(e) Unavailability of drug or device despite good faith compliance with WAC 246-869-150.
(2) Nothing in this section requires pharmacies to deliver a drug or device without payment of their usual and customary or contracted charge.
(3) If despite good faith compliance with WAC 246-869-150, the lawfully prescribed drug or device is not in stock, or the prescription cannot be filled pursuant to subsection (1)(a) of this section, the pharmacy shall provide the patient or agent a timely alternative for appropriate therapy which, consistent with customary pharmacy practice, may include obtaining the drug or device. These alternatives include but are not limited to:
(a) Contact the prescriber to address concerns such as those identified in subsection (1)(a) of this section or to obtain authorization to provide a therapeutically equivalent product;
(b) If requested by the patient or their agent, return unfilled lawful prescriptions to the patient or agent; or
(c) If requested by the patient or their agent, communicate or transmit, as permitted by law, the original prescription information to a pharmacy of the patient's choice that will fill the prescription in a timely manner.
(4) Engaging in or permitting any of the following shall constitute grounds for discipline or other enforcement actions:
(a) Destroy unfilled lawful prescription.
(b) Refuse to return unfilled lawful prescriptions.
(c) Violate a patient's privacy.
(d) Discriminate against patients or their agent in a manner prohibited by state or federal laws.
(e) Intimidate or harass a patient.
[]