WSR 16-16-016
PROPOSED RULES
DEPARTMENT OF HEALTH
[Filed July 21, 2016, 2:24 p.m.]
Original Notice.
Preproposal statement of inquiry was filed as WSR 13-09-041.
Title of Rule and Other Identifying Information: Chapter 246-226 WAC, Radiation protection—Computed tomography, establishes a new chapter of rules for the safe and effective use of computed tomography (CT) X-ray systems for diagnostic purposes.
Hearing Location(s): Department of Health, Town Center 3, Room 224, 111 Israel Road S.E., Tumwater, WA 98511, on September 7, 2016, at 10:00 a.m.
Date of Intended Adoption: September 14, 2016.
Submit Written Comments to: Michelle K. Austin, P.O. Box 47820, Olympia, WA 98504-7820, e-mail https://fortress.wa.gov/doh/policyreview, by September 7, 2016.
Assistance for Persons with Disabilities: Contact Michelle K. Austin by August 31, 2016, TTY (800) 833-6388 or 711.
Purpose of the Proposal and Its Anticipated Effects, Including Any Changes in Existing Rules: The proposed rule will establish requirements in a new chapter of rules for the safe and effective use of CT X-ray systems for diagnostic purposes.
Reasons Supporting Proposal: The use of CT technology has grown in recent years in the number of units, the frequency of prescribed scans, and most importantly, the amount of radiation used. The proposed rules are intended to reduce occupational and patient radiation exposure and help prevent overexposure incidents in Washington state that have occurred in other states in recent years.
Statute Being Implemented: Chapter 70.98 RCW.
Rule is not necessitated by federal law, federal or state court decision.
Name of Proponent: Department of health, governmental.
Name of Agency Personnel Responsible for Drafting, Implementation, and Enforcement: Dan Van Gent, 111 Israel Road S.E., Tumwater, WA 98511, (360) 236-3231.
A small business economic impact statement has been prepared under chapter 19.85 RCW.
Small Business Economic Impact Statement
SECTON [SECTION] 1: Describe the proposed rule, including: A brief history of the issue; an explanation of why the proposed rule is needed; and a brief description of the probable compliance requirements and the kinds of professional services that a small business is likely to need in order to comply with the proposed rule.
There are two hundred thirty-five hospitals and clinics (facilities) in Washington state using approximately four hundred CT X-ray systems. Currently, anyone using a CT X-ray system must register with the department of health (department) as required by chapters 70.98 RCW and 246-224 WAC, Radiation protection—Radiation machine assembly and registration. Under the generally applicable X-ray requirements for the healing arts established in chapter 246-225 WAC, Radiation protection—X rays in the healing arts, the department inspects registered CT X-ray systems for the health and safety of operators and the public. During inspections, the department notes if the registrant is accredited. If so, the department reviews the last medical physicist survey and records typical doses for head and body scans. The department is proposing rules to establish requirements in a new chapter for the safe and effective use of CT X-ray systems for diagnostic purposes. The proposed rules include requirements for facilities, equipment, staffing, operation and maintenance, records, and reporting requirements, which, collectively, are intended to reduce radiation exposure to the public and help prevent incidents of overexposure of patients and staff.
National Perspective: The use of CT technology has grown in recent years in the number of units, the frequency of prescribed scans, and most importantly, the amount of radiation used. In an October 8, 2009, Initial Communication, the United States Food and Drug Administration (FDA) acknowledged that two hundred six patients had been accidentally exposed to excess CT-generated radiation at the Cedars-Sinai Medical Center in California over an eighteen month period beginning February 2008. At least forty-four more CT-generated radiation overdose incidents were subsequently discovered at Glendale Adventist Medical Center and at Providence St. Joseph Medical Center in Burbank, California. A number of patients at Huntsville Hospital in Alabama were also exposed to excessive CT-generated radiation. The majority of these excess radiation exposures caused injurious adverse health effects. These findings resulted in adoption of strict rules for CT exams and procedures with stringent upper limits on acceptable radiation doses delivered during CT exams by the state of California radiation authority.
As CT technology advanced rapidly, professionals in the industry became aware that children were often times receiving standard adult CT-generated radiation doses. The doses were not adjusted for the smaller body sizes and shapes of pediatric patients and their increased sensitivity to radiation. Failure to adjust CT-generated radiation doses for children often results in radiation exposures three to four times greater than necessary for pediatric patients. For this and other reasons, several states in addition to California have created CT X-ray system rules including Oregon, Minnesota, Colorado, Utah, Michigan, Nebraska, and Ohio.
Further investigation by the FDA through 2010 revealed that approximately three hundred eighty-five patients nationwide were exposed to excess amounts of radiation during CT brain perfusion scans at six different hospitals. This finding resulted in the FDA adopting a nationwide initiative to reduce unnecessary radiation exposures resulting from CT and other X-ray imaging procedures.1 However, there are currently no federal rules for any type of patient CT imaging procedures using gantry-style CT X-ray systems.
1http://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/RadiationDoseReduction/ucm2007191.htm.
Washington State Perspective: In 2005, two professional medical physicists recognized as qualified experts by the department X-ray program found and reported CT patient safety concerns in forty-three facilities surveyed in our state. When compared to the American College of Radiology's (ACR) recommended dose index reference levels, sixty percent of the facilities had higher than recommended dose index values for CT head exams, and more than four percent of the facilities had higher than recommended adult abdomen CT dose index values.
In February 2012, at a CT seminar in Tacoma, one of the same two medical physicists pointed out to the audience of CT operators, radiologists, and hospital administrators that he personally was aware of two recent CT patient overexposures that occurred in our state. He went on to say that the state of Washington has no regulations controlling the use of CT.
The department found many of the conditions that could contribute to the findings described above during inspections of CT X-ray systems over an eighteen month period beginning in 2013. Examples of findings include inadequate attention to protocol password protection, no designation of a responsible radiologist to oversee protocol selection, and no guidance for retakes which may lead to overexposure.
Approach to Rule Making: On January 1, 2012, Centers for Medicare and Medicaid Services began requiring all nonhospital facilities using CT to be accredited by either the ACR or the Intersocietal Accreditation Commission in order to receive medicare reimbursement. This accreditation requirement leaves a gap in complete accreditation since it does not apply to hospitals and facilities that do not receive medicare reimbursement. The proposed rules will create consistent statewide requirements for all facilities using CT X-ray systems for diagnostic purposes that are compatible with medicare standards. By establishing CT X-ray system requirements in rule, the department seeks to improve patient and operator safety.
To develop the proposed rules, the department used a collaborative rule-making approach. The department developed an initial draft rule based on recommendations from an advisory committee made up of experts in the field of CT. The advisory committee was composed of a representative cross-section of doctors, radiologic technologies, radiation medical physicists, nurses, and hospital administrators from both urban and rural facilities. The advisory committee met six times over nineteen months beginning in July 2013. The department further refined the rules for proposal based on an extensive informal review and comment period held in July 2015.
SECTION 2: Identify which businesses are required to comply with the proposed rule using the North American Industry Classification System (NAICS) codes and what the minor cost thresholds are.
Table A
NAICS Code |
NAICS Business Description |
# of businesses in WA |
Minor Cost Threshold = 1% of Average Annual Payroll |
Minor Cost Threshold = .3% of Average Annual Receipts |
621512 |
Diagnostic Imaging Centers |
137 |
$9,336 |
$7,701 |
622110 |
General Medical and Surgical Hospital |
89 |
$722,465 |
$557,047 |
622111 |
Office of Physician (except mental health specialist) |
3,178 |
$11,602 |
$7,192 |
SECTION 3: Analyze the probable cost of compliance. Identify the probable costs to comply with the proposed rule, including: Cost of equipment, supplies, labor, professional services and increased administrative costs; and whether compliance with the proposed rule will cause businesses to lose sales or revenue.
The department surveyed all two hundred thirty-five registrants (entities with registered CT X-ray systems in the state). The department also surveyed three medical physicist groups that provide service to registrants in the state to assess the potential cost of the proposed rule. The survey asked questions about each of the proposed sections and asked participants to identify if they already comply with the proposed rules, or if not, to provide cost estimates to comply. Table 1 below shows the cumulative cost of the proposed rules for the twenty-two respondents. For more information about the specific rule sections, please refer to the significant analysis that the department developed for this proposed chapter.
Table 1: Cumulative Cost of the Proposed Rules |
|
Respondent 1 |
$1500 |
Respondent 2 |
0 |
Respondent 3 |
0 |
Respondent 4 |
0 |
Respondent 5 |
245 |
Respondent 6 |
2250 |
Respondent 7 |
1890 |
Respondent 8 |
15573 |
Respondent 9 |
0 |
Respondent 10 |
0 |
Respondent 11 |
1500 |
Respondent 12 |
2136 |
Respondent 13 |
2250 |
Respondent 14 |
19100 |
Respondent 15 |
0 |
Respondent 16 |
21820 |
Respondent 17 |
0 |
Respondent 18 |
560 |
Respondent 19 |
15450 |
Respondent 20 |
7480 |
Respondent 21 (ten sites) |
59288 |
Respondent 22 |
0 |
Total |
$151,402 |
Average cost for all respondents |
$6,882 |
Average for all respondents reporting costs |
$10,814 |
Average cost excluding respondent with 10 sites |
$7,086 |
The department assumes that no businesses will lose sales or revenue by implementing the proposed rules.
SECTION 4: Analyze whether the proposed rule may impose more than minor costs on businesses in the industry.
Minor cost threshold (1% payroll) |
$9,336 |
Minor cost threshold (3/10% of receipts) |
$7,192 |
As defined in chapter 19.85 RCW, and based on the information above, the proposed rule or portions of the proposed rules may impose more than minor costs on businesses in the industry. The remainder of this document meets the requirements of RCW 19.85.030 and 19.85.040.
SECTION 5: Determine whether the proposed rule may have a disproportionate impact on small businesses as compared to the ten percent of businesses that are the largest businesses required to comply with the proposed rule.
Based on cost estimates received from survey respondents, the department assumes many of the costs of the proposed rule are comparable regardless of business size. Therefore, the department assumes the proposed rules are likely to impose a disproportionate impact on small businesses.
SECTION 6: If the proposed rule has a disproportionate impact on small businesses, identify the steps taken to reduce the costs of the rule on small businesses. If the costs cannot be reduced provide a clear explanation of why.
Modifications to the proposed rules were made to balance the burden of reporting injuries with the necessity to improve patient safety and reduce the incidence of medical errors (CT events) that contribute to injuries. These changes include exempting registrants who include CT events in another department-approved coordinated quality assurance program from the event reporting requirements. The proposed exemption allows small business to take advantage of existing reporting processes rather than creating a new process for the purposes of the proposed rules alone.
Though the proposed rules follow national standards quite closely, they were modified to reduce staffing requirements established in national standards to balance patient safety with patient access to services in rural and acute access areas of the state. This is also an important modification to be consistent with department authority related to the regulation of health professionals.
Overall, the department is proposing rules that provide the least burdensome requirements that still protect occupational and public health and safety.
SECTION 7: Describe how small businesses were involved in the development of the proposed rule.
As described in Section 1: Approach to Rule Making, the department worked closely with an advisory committee to develop the proposed rules. The advisory committee included representatives from both urban and rural areas specifically to include small business perspectives in the development of the proposed rules. In addition, the advisory committee began development of the proposed rules from the basis of national standards and medicare reimbursement requirements specifically to create consistent statewide requirements for all facilities, including small businesses that currently receive medicare reimbursement for services and assumedly meet those requirements. The department further refined the rules for proposal based on an extensive informal review and comment period and cost survey which included all registrants and outreach to facilities in rural and acute access areas of the state.
SECTION 8: Identify the estimated number of jobs that will be created or lost as the result of compliance with the proposed rule.
The department assumes no jobs will be created or lost as a result of the proposed rules.
A copy of the statement may be obtained by contacting Michelle Austin, P.O. Box 47820, Olympia, WA 98504-7820, phone (360) 236-3250, e-mail Michelle.Austin@doh.wa.gov.
A cost-benefit analysis is required under RCW 34.05.328. A preliminary cost-benefit analysis may be obtained by contacting Michelle Austin, P.O. Box 47820, Olympia, WA 98504-7820, phone (360) 236-3250, e-mail Michelle.Austin@doh.wa.gov.
July 21, 2016
John Wiesman, DrPH, MPH
Secretary
Chapter 246-226 WAC
RADIATION PROTECTION—COMPUTED TOMOGRAPHY
NEW SECTION
WAC 246-226-001 Authority, purpose, and scope.
The requirements of this chapter are adopted pursuant to the provisions of chapter 70.98 RCW.
This chapter establishes CT X-ray system requirements for the intentional exposure of humans to ionizing radiation for diagnostic imaging.
NEW SECTION
WAC 246-226-006 Exemptions.
(1) Registrants exclusively using low power CT X-ray systems (5 kW or less) or cone-beam CT X-ray systems are exempt from the requirements of this chapter and shall comply with chapter 246-225 WAC. Cone-beam CT X-ray system is a variation of gantry-style CT X-ray system that rotates around the patient, capturing data using a cone-shaped X-ray beam. This data is used to reconstruct a three-dimensional image.
(2) Registrants using CT simulators exclusively for treatment planning purposes in conjunction with a megavoltage radiation therapy or brachytherapy is exempt from the requirements of this chapter, except that the registrant shall comply with WAC 246-226-090, 246-240-113, and chapter 246-225 WAC.
NEW SECTION
WAC 246-226-007 Relationship to other regulations.
In addition to the requirements established in this chapter, registrants shall also comply with applicable requirements of the following:
(1) Chapter 246-220 WAC;
(2) Chapter 246-221 WAC;
(3) Chapter 246-222 WAC;
(4) Chapter 246-224 WAC;
(5) Chapter 246-225 WAC; and
(6) Chapter 246-254 WAC.
NEW SECTION
WAC 246-226-010 Definitions, abbreviations, and acronyms.
The definitions, abbreviations, and acronyms in this section, WAC 246-220-010 and 246-225-010, apply throughout this chapter unless the context clearly indicates otherwise.
(1) "CT" or "computed tomography" means technology that uses computer-processed X rays to produce tomographic images (virtual slices) of specific areas of the patient's body or scanned object.
(2) "CTDI" or "computed tomography dose index" means the integral of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single scan that is:
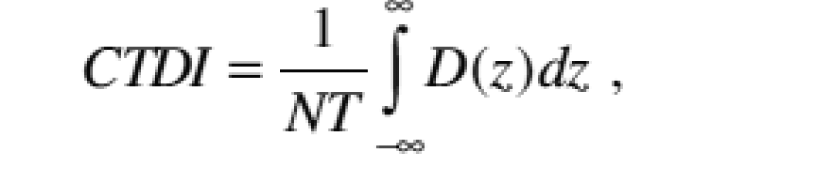 |
Where: |
|
Z = Position along a line perpendicular to the tomographic plane; |
|
D(z) = Dose at position z; |
|
T = Nominal tomographic section thickness; |
|
N = Number of tomograms produced in a single scan. |
|
And: |
|
The dose profile is centered around z and that, for a multiple tomogram system, the scan increment between adjacent scans is nT. |
|
(3) "CTDIvol" means the product of the CTDIw and NT, divided by the table increment I and expressed as milliGray.
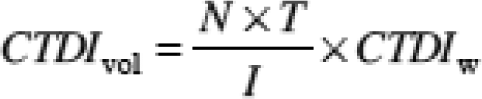 |
(4) "CT dosimetry phantom" means an object used to determine the dose delivered by a CT X-ray system.
(5) "CTN" or "computed tomography number" means the number used to represent the X-ray attenuation associated with each elemental area of the CT image:
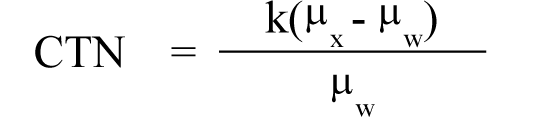 |
Where: |
|
k = A constant, a normal value of 1,000 when the Hounsfield scale of CTN is used; |
|
μx = Linear attenuation coefficient of the material of interest; |
|
μw = Linear attenuation coefficient of water. |
|
(6) "CT procedure" means an activity directed at or performed on a patient necessary to make a diagnosis using a CT X-ray system including, but not limited to, setting, modifying, or applying parameters or protocols.
(7) "CT simulator" means a CT unit that allows for precise cancer treatment planning by demonstrating the relationship between the target tumor and healthy tissues while the patient is in a treatment position.
(8) "CT X-ray system" means a gantry-style X-ray system that generates a tomographic image through acquisition of cross-sectional image slices perpendicular to the plane of travel of the gantry.
(9) "Department" means the Washington state department of health.
(10) "Dose profile" means the dose as a function of position along a line.
(11) "DLP" or "dose length product" means the product of the CTDIvol and the scan length of a single or group of scans performed on the same body part. This number can be calculated over the entire CT procedure to give an estimate of the total dose. The value is expressed in milliGray centimeters.
(12) "Filtration" means material placed in the beam to preferentially absorb low energy photons that contribute no diagnostically meaningful data to the image.
(13) "Fixed CT X-ray system" means a CT X-ray system that is permanently mounted in the building in which it is used or a portable CT X-ray system that is permanently stationed in one location.
(14) "Joint commission" means the independent, not-for-profit organization that accredits and certifies health care organizations and programs in the United States.
(15) "kW" or "kilowatts" means peak power, which is the highest rated kilovoltage of a CT X-ray system multiplied by the maximum rated amperage multiplied by the power factor.
(16) "Lead CT technologist" means the radiologic technologist licensed under chapter 18.84 RCW and designated by the registrant to perform the duties identified in this chapter. A licensed health care professional listed in RCW 18.130.040 acting within their scope of practice may also be designated by the registrant to perform the duties identified in this chapter.
(17) "Lead interpreting CT physician" means a physician licensed under chapter 18.71 or 18.57 RCW designated by the registrant to perform the duties identified in this chapter. A licensed health care professional listed in RCW 18.130.040 acting within their scope of practice may also be designated by the registrant to perform the duties identified in this chapter.
(18) "Mobile CT X-ray system" means a CT X-ray system that is permanently mounted in a vehicle or trailer.
(19) "Noise" means the standard deviation of the fluctuations in CTN expressed as a percentage of the attenuation coefficient of water. Its estimate (Sn) is calculated using the following expression:
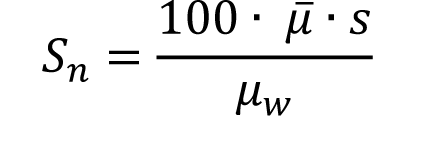 |
Where: |
|
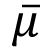 = Linear attenuation coefficient of the material of interest; = Linear attenuation coefficient of the material of interest; |
|
μw = Linear attenuation coefficient of water; |
|
s = Standard deviation of the CTN of picture elements in a specified area of the CT image. |
|
(20) "Nominal tomographic section thickness" means the full width at half-maximum of the sensitivity profile taken at the center of the cross-sectional volume over which X-ray transmission data are collected.
(21) "Operator" means a Washington state licensed health care professional whose scope of practice includes CT diagnostics which includes choosing the appropriate scan protocol, appropriately adjusting parameters when necessary, and administering the CT procedure.
(22) "PACS" or "picture archiving and communication system" means a medical imaging technology that provides economical storage of and convenient access to images from CT.
(23) "Parameter" means settings on the CT X-ray system that can be modified including, but not limited to, peak tube potential in kV, filtration thickness, the tube current in mA and the exposure time in milliseconds, and the product of tube current and exposure time in mAs.
(25) "Portable CT X-ray system" means a CT X-ray system that is not permanently mounted in a building, vehicle, or trailer and is able to move between locations of use.
(26) "Protocol" means the collection of settings and parameters affecting CT dose and image quality that specify how data collection and reconstruction, patient positioning, and contrast administration are performed.
(27) "Qualified medical physicist" means a physicist who meets the requirements of WAC 246-226-065.
(28) "Radiologic technologist" means an individual licensed under chapter 18.84 RCW.
(29) "Scan" means the complete process of collecting X-ray transmission data for the production of a tomogram.
(30) "Scan increment" means the amount of relative displacement of the patient with respect to the CT X-ray system between successive scans measured along the direction of such displacement.
(31) "Sensitivity profile" means the relative response of the CT X-ray system as a function of position along a line perpendicular to the tomographic plane.
(32) "SSDE" or "size specific dose estimate" means a patient dose estimate which takes into consideration corrections based on the size of the patient using linear dimensions measured on the patients or the patient images.
(33) "Tomogram" means a two dimensional image representing a slice or section through a three dimensional object using a CT X-ray system.
(34) "Tomographic plane" means the geometric plane which is identified as corresponding to the tomogram.
(35) "Tomographic section" means the volume of an object whose X-ray attenuation properties are imaged in a tomogram.
NEW SECTION
WAC 246-226-020 Equipment requirements.
The CT X-ray system must:
(1) Meet the requirements of 21 C.F.R. Sec. 1020.33 (last amended June 10, 2005) at the time of installation and while the CT X-ray system is registered with the department under chapter 246-224 WAC; and
(2) Be equipped:
(a) With a visible signal that indicates when the X-ray exposure is occurring;
(b) So that the operator can immediately terminate an X-ray exposure of greater than one-half second duration at any time during the X-ray exposure;
(c) So that the parameters used during a CT procedure are:
(i) Displayed prior to beginning a scan; and
(ii) Visible by the operator from any location scanning can be initiated.
(d) So that radiation leakage from the tube port does not exceed limits established in WAC 246-225-040 (3) and (4) when data are not being collected for image production.
(e) So that the accuracy of the laser or optical positioning system is within five millimeters maximum deviation on the axial position (z-axis).
(f) With an X-ray production indicator of at least one-half second at or near the gantry that is visible from any point outside the gantry opening.
(g) So that premature termination of the X-ray exposure by the operator requires resetting of the parameters prior to initiating another scan.
NEW SECTION
WAC 246-226-030 Design requirements.
(1) The location of a CT X-ray system must be designed and constructed:
(a) To provide for two-way verbal communication between the patient and the operator at the control panel;
(b) To allow the operator to continuously observe the patient from the control panel during irradiation using windows, mirrors, closed-circuit television, or an equivalent method; and
(c) With an alternate viewing system when the primary viewing system is electronic.
(2) After (insert effective date of chapter) and within thirty days of first use of a CT X-ray system, the registrant shall complete and keep on file a radiation protection survey of the room and surrounding areas consistent with National Council on Radiation Protection and Measurements Report #147 (2004). For CT X-ray systems in use before (insert effective date of the chapter), the registrant shall complete the radiation protection survey by (two years after effective date of the chapter) and keep on file.
(3) For fixed or mobile CT X-ray systems:
(a) Installed after (effective date of the rule), the operator's booth and surrounding occupied areas must be designed and constructed in accordance with the National Council on Radiation Protection and Measurements Report #147 (2004);
(b) Protective barriers must be provided in the ceiling, floor, and walls of the CT X-ray system enclosure to ensure exposure does not exceed dose limits established in chapter 246-221 WAC; and
(c) The control panel must be shielded by a protective barrier that cannot be removed from a protective position between the operator and the radiation source during CT X-ray system operation.
(4) The registrant shall submit a revised radiation shielding plan for department review in accordance with WAC 246-225-030 after replacement of the CT X-ray system, or any change in the CT X-ray system room's construction or surrounding rooms' construction.
(5) Rooms in which a portable CT X-ray system is used are exempt from the requirements of subsections (2), (3), and (4) of this section. However, the operator must be protected to ensure exposure does not exceed dose limits established in chapter 246-221 WAC.
NEW SECTION
WAC 246-226-040 Operating procedures.
(1) The registrant shall:
(a) Establish a procedure to record and retrieve CTDIvol, DLP and, when available on the CT X-ray system, SSDE data from every CT procedure performed; and
(b) If available, send each protocol page that lists the technique factors electronically to the PACS.
(2) The registrant shall provide estimated patient dose for an individual study within ten business days of a patient request.
(3) Effective (insert date six months after the effective date of these rules), the registrant shall establish CT procedures for each CT X-ray system in consultation with a qualified medical physicist and the lead interpreting CT physician, or lead CT technologist to ensure they are correct for the intended dose and image quality. The CT procedures must include:
(a) Pediatric CT protocols for each CT X-ray system used for pediatric patients.
(b) Procedural, software, and engineering measures that prohibit anyone from changing protocols or parameters without approval from the lead CT technologist or the lead interpreting CT physician, such as password protection.
(c) Documentation of protocol or parameter changes must be maintained consistent with the requirements of WAC 246-226-100.
(4) The registrant may not allow the CT manufacturer's technical or applications representatives to make protocol changes or other software changes or upgrades that would impact radiation dose or image quality without the approval of the lead interpreting CT physician, the lead CT technologist, or the qualified medical physicist.
(5) Effective (insert date twelve months after the effective date of these rules), the registrant shall review CT protocols in consultation with a qualified medical physicist and the lead interpreting CT physician, or lead CT technologist to ensure they are correct for the intended dose and image quality as follows:
(a) Review all CT protocols upon installation of a CT X-ray system;
(b) Annually review the following protocols:
(i) New or changed protocols since the last review;
(ii) Pediatric head;
(iii) Pediatric abdomen;
(iv) Adult head;
(v) Adult abdomen;
(vi) High resolution chest; and
(vii) Brain perfusion.
(c) If the facility does not perform the procedures listed in (b)(ii) through (vii) of this subsection, the registrant shall annually review the most frequently performed or highest dose protocols so that a total of at least six protocols are reviewed annually.
(d) As part of the review, the registrant shall:
(i) Compare current protocols to the dose assessments that were made during the last annual performance evaluation required in WAC 246-226-090;
(ii) Determine whether the protocols from each CT procedure are appropriate, can be modified to lower the CTDIvol without an unacceptable sacrifice in image quality, or can be eliminated;
(iii) Establish protocols to maintain image quality at the optimal noise level (standard deviation) within dose levels established in WAC 246-226-050.
(iv) Establish guidelines of variability that establish parameter and protocol limits.
(6) The registrant shall limit the use of the CT X-ray system to those permitted by the established guidelines of variability.
(7) The operator may adjust parameters or protocols for a CT procedure as long as they remain within the approved limits established in the guidelines of variability.
(8) The operator shall check the display panel before and after performing each scan to make sure the amount of radiation delivered is appropriate for the CT procedure and individual patient. This may be accomplished by reviewing dose indicator devices if available or dose indices. The operator shall document dose indicators or indices outside expected values and submit the documentation to the lead interpreting CT physician or qualified medical physicist for review.
(9) Each registrant shall create a written policy establishing procedures for retaking CT scans including, but not limited to, how many scans are authorized for a patient and who can authorize additional retakes. The policy must be approved by the lead interpreting CT physician.
(10) When a patient must be held in position for a CT procedure, mechanical supporting or restraining devices must be used unless contraindicated. If the patient must be held by an individual, the individual shall:
(a) Wear protective gloves and a protective apron of at least 0.5 millimeter lead equivalent;
(b) Be positioned so that no part of his or her body will be struck by the useful beam; and
(c) Be positioned so that his or her body is as far as possible from the edge of the useful beam.
(11) If staff routinely working with or around radiation sources hold patients during CT procedures, personnel exposure may not exceed the dose limits established in chapter 246-221 WAC.
(12) Only individuals whose presence is necessary are allowed in a CT X-ray system room during exposure. Each individual, except the patient, shall be protected by at least 0.5 millimeter lead equivalent apron or a whole body protective barrier.
NEW SECTION
WAC 246-226-050 Dose limits.
The CTDIvol for the following CT procedure on phantoms may not exceed the dose limits established in Table 1.
Table 1: Dose limits
CT Procedure |
Phantom Size |
Dose Limit: CTDIvol (mGy) |
Adult head |
16 cm |
80 |
Adult abdomen |
32 cm |
30 |
Pediatric head (one year old) |
16 cm |
40 |
Pediatric abdomen (40 pounds) |
16 cm |
20 |
NEW SECTION
WAC 246-226-060 CT events.
(1) The purpose of this section is to improve patient safety by supporting health care providers and facilities in their efforts to reduce the incidence of medical errors that contribute to deterministic injurious health effects. This rule does not relieve the department of its statutory obligation to enforce this and other radiation protection laws.
(2) The registrant shall initiate an investigation within twenty-four hours and complete the investigation within ten business days when:
(a) The cumulative CTDIvol over the course of an individual study at a particular anatomical location exceeds 600 mGy for a pediatric CT procedure or 1500 mGy for an adult CT procedure; or
(b) Any ionizing radiation exposure from a CT procedure results in unanticipated hair loss, erythema, or functional damage to an organ or physiological system.
(3) For each event, the registrant shall conduct a root cause analysis in consultation with a qualified medical physicist, the lead interpreting CT physician, lead CT technologist, and the operator who performed the CT procedure. The root cause analysis must:
(a) Follow the procedures and methods of:
(i) The joint commission;
(ii) The department of veterans affairs national center for patient safety; or
(iii) A department-approved nationally recognized root cause analysis methodology.
(b) Include the following information:
(i) The findings regarding the root cause of the event;
(ii) The number and types of health professionals present at the time the reported event occurred;
(iii) A corrective action plan consistent with the findings of the root cause analysis and including:
(A) How each finding will be addressed and corrected;
(B) When each correction will be completed;
(C) Who is responsible to implement the corrections;
(D) What action will be taken to prevent the event from recurring; and
(iv) A monitoring schedule to assess the effectiveness of the corrective action plan, including who is responsible for the monitoring schedule.
(c) If the registrant determines there is no need to create a corrective action plan for a particular event, include a written explanation for the determination.
(4) The root cause analysis must not include any identifying information for any health care professional, facility employee, or patient involved.
(5) The registrant shall make appropriate modifications consistent with the corrective action plan to prevent future events.
(6) This rule does not remove a registrant's responsibility to report a licensed practitioner's unprofessional conduct to the department, as defined under RCW 18.130.180.
(7) A registrant is exempt from the requirements of this section when the registrant is subject to other coordinated quality improvement requirements under RCW 70.41.200 or 70.230.080, and includes CT events as part of the required coordinated quality improvement program.
(8) A registrant is exempt from the requirements of this section when the registrant includes CT events as part of a department-approved coordinated quality improvement program under RCW 43.70.510.
NEW SECTION
WAC 246-226-065 Qualified medical physicist.
A qualified medical physicist must meet the requirements of either subsection (1), (2), or (3) of this section, and meet the continuing education and experience requirements of subsections (4) and (5) of this section to perform the duties of a qualified medical physicist. To qualify as a qualified medical physicist, an individual must:
(1) Hold a valid certificate in:
(a) Diagnostic radiological physics or radiological physics from the American Board of Radiology;
(b) Diagnostic imaging physics from the American Board of Medical Physics; or
(c) Diagnostic radiology physics from the Canadian College of Physicists in Medicine.
(2) Complete the following education and experience:
(a) Graduate from an accredited institution with a graduate degree in medical physics, radiological physics, physics, or another relevant physical science or engineering discipline; including formal course work in the biological sciences with at least:
(i) One course in biology or radiation biology; and
(ii) One course in anatomy, physiology, or similar topics related to the practice of medical physics.
(b) Three years of documented experience in a clinical CT environment.
(3) Independently evaluated at least three CT X-ray systems in accordance with this chapter in the three years prior to (the effective date of this chapter).
(4) Continuing education:
(a) The qualified medical physicist must have earned at least fifteen continuing medical education units in the three years preceding any department review or inspection.
(i) At least half the units must be accredited by the Accreditation Council for Continuing Medical Education or equivalent accreditation; and
(ii) At least one of the units must pertain to CT.
(b) The requirements of this subsection are waived if it has been less than three years since the qualified medical physicist met the requirements under subsection (2) of this section.
(5) Continuing experience: The qualified medical physicist must meet (a) or (b) of this subsection in the two years preceding any department review or inspection:
(a) Independently evaluated at least two CT X-ray systems in accordance with this chapter; or
(b) Evaluated at least five CT X-ray systems in accordance with this chapter under the direct supervision of a qualified medical physicist;
(c) The requirements of this subsection are waived if it has been less than two years since the qualified medical physicist met the requirements of subsection (2) or (3) of this section.
NEW SECTION
WAC 246-226-070 Staffing requirements.
(1) Each registrant with a CT X-ray system shall employ or contract with a licensed health care professional to perform CT procedures. Nothing in this chapter prohibits the registrant from requiring the employed or contracted radiologic technologist to hold a recognized national certification or other accreditation relevant to CT, such as an American Registry of Radiologic Technologists (ARRT) CT certification, prior to performing CT procedures.
(2) The registrant shall provide training to operators performing CT procedures within six months of employment and annually thereafter. Training must be documented for all radiologic technologists in a single training log. The training must be equivalent to the International Atomic Energy Agency, 10 Pearls: Radiation Protection of Patients in CT. This document is located on the department's web site at http://www.doh.wa.gov/CommunityandEnvironment/Radiation/XRay
(3) Each registrant with a CT X-ray system shall employ or contract with a qualified medical physicist and verify the qualified medical physicist meets the requirements of WAC 246-226-065 to perform the activities of the qualified medical physicist specified in this chapter.
(4) The registrant shall appoint a lead interpreting CT physician and a lead CT technologist to work cooperatively to:
(a) Develop, implement, and enforce policies, procedures, and registrant requirements that address:
(i) Radiation protection, the hazards of radiation exposure to both patients and facility personnel, and appropriate monitoring;
(ii) Identification of pregnant or potentially pregnant patients; and
(iii) Safety issues, including contrast use and sedation, and reducing exposure as much as reasonably possible for pediatric patients;
(b) Ensure that a physician is present and immediately available when contrast is administered to a patient; and
(c) Be responsible for:
(i) Implementing the quality control program required in WAC 246-226-080;
(ii) Ensuring compliance with the recommendations of the qualified medical physicist; and
(iii) Overseeing all CT-related materials including, but not limited to, clinical and phantom images, quality control data, and other information required by this chapter.
NEW SECTION
WAC 246-226-080 Quality control program.
(1) The registrant shall establish, document, and implement a quality control program in consultation with the qualified medical physicist before using a CT X-ray system. The quality control program must be consistent with the manufacturer's recommended quality control standards if available. The quality control program must include, but is not limited to, the following:
(a) On each day of clinical use, measurement of water CTN and standard deviation;
(b) On each day of clinical use, artifact evaluation;
(c) For registrants using wet laser hardcopy for primary interpretation, weekly printer quality control;
(d) Monthly visual checklist;
(e) For registrants using dry laser hardcopy for primary interpretation, monthly printer quality control; and
(f) Monthly display monitors quality control.
(2) If the results of an evaluation included in the quality control program do not meet the requirements of this chapter, the registrant, in consultation with the qualified medical physicist, shall modify the quality control program and document the changes.
NEW SECTION
WAC 246-226-090 Performance evaluation.
The qualified medical physicist shall conduct a performance evaluation to assess the quality and safety of the CT X-ray system and its operation.
(1) A performance evaluation must be conducted:
(a) Within thirty days of installation if the CT X-ray system passes all manufacture installation tests;
(b) Annually following the initial evaluation; and
(c) After any change, replacement, or reconfiguration of components which, in the opinion of the qualified medical physicist, could cause a change in the radiation output or image quality.
(2) A performance evaluation must evaluate:
(a) Alignment light accuracy;
(b) Slice localization from scanned projection radiograph;
(c) Table increment and travel accuracy;
(d) Slice thickness accuracy;
(e) Image quality, including the following:
(i) High-contrast resolution;
(ii) Low-contrast resolution;
(iii) Image uniformity;
(iv) Noise; and
(v) Artifact evaluation.
(f) Gray level performance of CT acquisition display monitors;
(g) CTN uniformity, accuracy, and linearity;
(h) Safety, including the following:
(i) Visual inspection;
(ii) Audible and visual signals; and
(iii) Posting requirements.
(i) The ongoing quality control program under WAC 246-226-080, including evaluation results and corrective actions;
(j) Protocols;
(k) Radiation output by:
(i) Using a calibrated dosimetry system that:
(A) Has been calibrated within the preceding twenty-four months; and
(B) Is traceable to a national standard.
(ii) Using a CT dosimetry phantom that:
(A) Is a right circular cylinder of polymethyl methacrylate of density 1.19 plus or minus 0.01 grams per cubic centimeter;
(B) Is at least 14 centimeters in length;
(C) Is 32.0 centimeters in diameter for evaluating CT X-ray systems designed to image any section of the body;
(D) Is 16.0 centimeters for systems designed to image the head, or for whole body CT X-ray systems operated in the head scanning mode; and
(E) Provides for the placement of a dosimeter along the axis of rotation and along a line parallel to the axis of rotation 1.0 centimeters from the outer surface and within the phantom. The qualified medical physicist may place additional dosimeters or alignment devices at other locations.
(iii) Performing all dose assessments with the CT dosimetry phantom placed on the patient support device without additional attenuation materials present;
(iv) Measuring the CTDIvol by orienting the CT dosimetry phantom so that the measurement point 1.0 centimeter from the peripheral outer surface of the phantom and the measurement point along the axial line of the phantom is in the same angular position within the gantry as the point of maximum surface CTDIvol identified. The parameters must correspond to typical values used for the average patient protocol. For the purpose of determining the CTDIvol, the manufacturer's nominal tomographic section thickness for that particular CT X-ray system may be used.
(l) Accuracy of the displayed dose on the CT X-ray system console and verify the displayed dose is within twenty percent of the measured dose.
(3) The qualified medical physicist shall prepare a performance evaluation report and provide it to the registrant within thirty days of completing the performance evaluation. The report must include:
(a) A summary of the performance evaluation required under this section.
(b) Recommendations for improvements, if any.
(c) Type of radiation detection instrument or system used, including the date of the last calibration.
NEW SECTION
WAC 246-226-100 Required records and reports.
(1) The registrant shall maintain written information regarding the operation and calibration of the CT X-ray system including, but not limited to, the following:
(a) Dates of the latest calibration and where the results are located; and
(b) Quality control program results required under WAC 246-226-080 of at least the most recent quality control evaluation, and all additional schedules of evaluation established by the qualified medical physicist appropriate for the CT X-ray system.
(2) A registrant shall maintain the following documents for the durations specified and make them available for review by the department upon request:
(a) The most recent radiation protection survey and radiation shielding plan required under WAC 246-226-030 must be retained for as long as the CT X-ray system is in use.
(b) Written approval of the most recent annual review under WAC 246-226-040 for each CT X-ray system with date and signature of the registrant, qualified medical physicist, and lead interpreting CT physician.
(c) Most recent performance evaluation report required under WAC 246-226-090.
(d) Records documenting the qualifications of all personnel who worked with the CT X-ray system as a physician, radiologic technologist, and employed or contracted qualified medical physicist, during the past three years. Records of personnel no longer employed by the CT facility must be retained until the next department inspection following the employee's termination has been completed and the department has determined that the facility is in compliance with the staffing requirements of WAC 246-226-070.
(e) Training log required under WAC 246-226-070, must be retained for at least three years.
(f) All root cause analyses and corrective actions plans required under WAC 246-226-060, must be retained for at least ten years.
NEW SECTION
WAC 246-226-110 Variance request.
A registrant may submit a written request to the department for a variance from the applicable regulations. The registrant shall not use CT X-ray system on patients until the department approves the variance request.
(1) The written request shall be addressed to: X-ray Supervisor, Office of Radiation Protection, Department of Health, P.O. Box 47827, Olympia, WA 98504-7827, and must include:
(a) The specific WAC reference or references of the rule for which the variance is requested;
(b) An explanation of the circumstances involved, and the reason why the rule cannot be followed;
(c) A description of how the proposed alternative meets the intent of the rule and how the registrant shall protect patients, employees, and the public;
(d) A description of the CT X-ray system to be used with supporting pictures or documents; and
(e) The time period for which the variance is requested.
(2) The department may grant a variance if it determines the variance is authorized by law and will not result in undue hazard to public health and safety or property. The department may impose conditions that are necessary to protect human health and safety during the term of the variance.
(3) The department may require the registrant to submit additional information.
(4) The department may conduct an on-site variance inspection to verify the information provided or if it determines that an inspection is necessary.
(5) As determined by the department, variances can be permanent or temporary.
(6) The department may at any time revoke a variance if it is determined that the conditions of the variance are not being followed.