Method number: ID-191
Matrix: Bulk
Collection Procedure
Collect approximately 1 to 2 grams of each type of material and place into separate 20 mL scintillation vials.
Analytical Procedure
A portion of each separate phase is analyzed by gross examination, phase-polar examination, and central stop dispersion microscopy.
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-WISHA. Similar products from other sources may be substituted.
(1) Introduction
This method describes the collection and analysis of asbestos bulk materials by light microscopy techniques including phase-polar illumination and central-stop dispersion microscopy. Some terms unique to asbestos analysis are defined below:
Amphibole: A family of minerals whose crystals are formed by long, thin units which have two thin ribbons of double chain silicate with a brucite ribbon in between. The shape of each unit is similar to an "I beam." Minerals important in asbestos analysis include cummingtonite-grunerite, crocidolite, tremolite-actinolite and anthophyllite.
Asbestos: A term for naturally occurring fibrous minerals. Asbestos includes chrysotile, cummingtonite-grunerite asbestos (amosite), anthophyllite asbestos, tremolite asbestos, crocidolite, actinolite asbestos and any of these minerals which have been chemically treated or altered. The precise chemical formulation of each species varies with the location from which it was mined. Nominal compositions are listed:
Chrysotile. . . . Mg3Si2O5(OH)4 | |
Crocidolite (Riebeckite asbestos). . . . Na2Fe32+Fe23+Si8O22(OH)2 | |
Cummingtonite-Grunerite asbestos (Amosite). . . . (Mg,Fe)7Si8O22(OH)2 | |
Tremolite-Actinolite asbestos. . . . Ca2(Mg,Fe)5Si8O22(OH)2 | |
Anthophyllite asbestos. . . . (Mg,Fe)7Si8O22(HO)2 | |
Asbestos Fiber: | A fiber of asbestos meeting the criteria for a fiber. (See section (3)(e).) |
Aspect Ratio: | The ratio of the length of a fiber to its diameter usually defined as "length: width", e.g. 3:1. |
Brucite: | A sheet mineral with the composition mg(OH)2. |
Central Stop Dispersion Staining (microscope): This is a dark field microscope technique that images particles using only light refracted by the particle, excluding light that travels through the particle unrefracted. This is usually accomplished with a McCrone objective or other arrangement which places a circular stop with apparent aperture equal to the objective aperture in the back focal plane of the microscope.
Cleavage Fragments: Mineral particles formed by the comminution of minerals, especially those characterized by relatively parallel sides and moderate aspect ratio.
Differential Counting: The term applied to the practice of excluding certain kinds of fibers from a phase contrast asbestos count because they are not asbestos.
Fiber: A particle longer than or equal to 5 microns with a length to width ratio greater than or equal to 3:1. This may include cleavage fragments. (See section (3)(e) of this appendix).
Phase Contrast: Contrast obtained in the microscope by causing light scattered by small particles to destructively interfere with unscattered light, thereby enhancing the visibility of very small particles and particles with very low intrinsic contrast.
Phase Contrast Microscope: A microscope configured with a phase mask pair to create phase contrast. The technique which uses this is called Phase Contrast Microscopy (PCM).
Phase-Polar Analysis: This is the use of polarized light in a phase contrast microscope. It is used to see the same size fibers that are visible in air filter analysis. Although fibers finer than 1 micron are visible, analysis of these is inferred from analysis of larger bundles that are usually present.
Phase-Polar Microscope: The phase-polar microscope is a phase contrast microscope which has an analyzer, a polarizer, a first order red plate and a rotating phase condenser all in place so that the polarized light image is enhanced by phase contrast.
Sealing Encapsulant: This is a product which can be applied, preferably by spraying, onto an asbestos surface which will seal the surface so that fibers cannot be released.
Serpentine: A mineral family consisting of minerals with the general composition Mg3(Si2O5(OH)4 having the magnesium in brucite layer over a silicate layer. Minerals important in asbestos analysis included in this family are chrysotile, lizardite, antigorite.
(a) History
Light microscopy has been used for well over 100 years for the determination of mineral species. This analysis is carried out using specialized polarizing microscopes as well as bright field microscopes. The identification of minerals is an on-going process with many new minerals described each year. The first recorded use of asbestos was in Finland about 2500 B.C. where the material was used in the mud wattle for the wooden huts the people lived in as well as strengthening for pottery. Adverse health aspects of the mineral were noted nearly 2000 years ago when Pliny the Younger wrote about the poor health of slaves in the asbestos mines. Although known to be injurious for centuries, the first modern references to its toxicity were by the British Labor Inspectorate when it banned asbestos dust from the workplace in 1898. Asbestosis cases were described in the literature after the turn of the century. Cancer was first suspected in the mid 1930's and a causal link to mesothelioma was made in 1965. Because of the public concern for worker and public safety with the use of this material, several different types of analysis were applied to the determination of asbestos content. Light microscopy requires a great deal of experience and craft. Attempts were made to apply less subjective methods to the analysis. X-ray diffraction was partially successful in determining the mineral types but was unable to separate out the fibrous portions from the nonfibrous portions. Also, the minimum detection limit for asbestos analysis by X-ray diffraction (XRD) is about 1%. Differential Thermal Analysis (DTA) was no more successful. These provide useful corroborating information when the presence of asbestos has been shown by microscopy; however, neither can determine the difference between fibrous and nonfibrous minerals when both habits are present. The same is true of Infrared Absorption (IR).
When electron microscopy was applied to asbestos analysis, hundreds of fibers were discovered present too small to be visible in any light microscope. There are two different types of electron microscopes used for asbestos analysis: Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM). Scanning Electron Microscopy is useful in identifying minerals. The SEM can provide two of the three pieces of information required to identify fibers by electron microscopy: Morphology and chemistry. The third is structure as determined by Selected Area Electron Diffraction-SAED which is performed in the TEM. Although the resolution of the SEM is sufficient for very fine fibers to be seen, accuracy of chemical analysis that can be performed on the fibers varies with fiber diameter in fibers of less than 0.2 micron diameter. The TEM is a powerful tool to identify fibers too small to be resolved by light microscopy and should be used in conjunction with this method when necessary. The TEM can provide all three pieces of information required for fiber identification. Most fibers thicker than 1 micron can adequately be defined in the light microscope. The light microscope remains as the best instrument for the determination of mineral type. This is because the minerals under investigation were first described analytically with the light microscope. It is inexpensive and gives positive identification for most samples analyzed. Further, when optical techniques are inadequate, there is ample indication that alternative techniques should be used for complete identification of the sample.
(b) Principle
Minerals consist of atoms that may be arranged in random order or in a regular arrangement. Amorphous materials have atoms in random order while crystalline materials have long range order. Many materials are transparent to light, at least for small particles or for thin sections. The properties of these materials can be investigated by the effect that the material has on light passing through it. The six asbestos minerals are all crystalline with particular properties that have been identified and cataloged. These six minerals are anisotropic. They have a regular array of atoms, but the arrangement is not the same in all directions. Each major direction of the crystal presents a different regularity. Light photons travelling in each of these main directions will encounter different electrical neighborhoods, affecting the path and time of travel. The techniques outlined in this method use the fact that light traveling through fibers or crystals in different directions will behave differently, but predictably. The behavior of the light as it travels through a crystal can be measured and compared with known or determined values to identify the mineral species. Usually, Polarized Light Microscopy (PLM) is performed with strain-free objectives on a bright-field microscope platform. This would limit the resolution of the microscope to about 0.4 micron. Because WISHA requires the counting and identification of fibers visible in phase contrast, the phase contrast platform is used to visualize the fibers with the polarizing elements added into the light path. Polarized light methods cannot identify fibers finer than about 1 micron in diameter even though they are visible. The finest fibers are usually identified by inference from the presence of larger, identifiable fiber bundles. When fibers are present, but not identifiable by light microscopy, use either SEM or TEM to determine the fiber identity.
(c) Advantages and Disadvantages
The advantages of light microscopy are:
(i) Basic identification of the materials was first performed by light microscopy and gross analysis. This provides a large base of published information against which to check analysis and analytical technique.
(ii) The analysis is specific to fibers. The minerals present can exist in asbestiform, fibrous, prismatic, or massive varieties all at the same time. Therefore, bulk methods of analysis such as X-ray diffraction, IR analysis, DTA, etc. are inappropriate where the material is not known to be fibrous.
(iii) The analysis is quick, requires little preparation time, and can be performed on-site if a suitably equipped microscope is available.
The disadvantages are:
(iv) Even using phase-polar illumination, not all the fibers present may be seen. This is a problem for very low asbestos concentrations where agglomerations or large bundles of fibers may not be present to allow identification by inference.
(v) The method requires a great degree of sophistication on the part of the microscopist. An analyst is only as useful as his mental catalog of images. Therefore, a microscopist's accuracy is enhanced by experience. The mineralogical training of the analyst is very important. It is the basis on which subjective decisions are made.
(vi) The method uses only a tiny amount of material for analysis. This may lead to sampling bias and false results (high or low). This is especially true if the sample is severely inhomogeneous.
(vii) Fibers may be bound in a matrix and not distinguishable as fibers so identification cannot be made.
(d) Method Performance
(i) This method can be used for determination of asbestos content from 0 to 100% asbestos. The detection limit has not been adequately determined, although for selected samples, the limit is very low, depending on the number of particles examined. For mostly homogeneous, finely divided samples, with no difficult fibrous interferences, the detection limit is below 1%. For inhomogeneous samples (most samples), the detection limit remains undefined. NIST has conducted proficiency testing of laboratories on a national scale. Although each round is reported statistically with an average, control limits, etc., the results indicate a difficulty in establishing precision especially in the low concentration range. It is suspected that there is significant bias in the low range especially near 1%. EPA tried to remedy this by requiring a mandatory point counting scheme for samples less than 10%. The point counting procedure is tedious, and may introduce significant biases of its own. It has not been incorporated into this method.
(ii) The precision and accuracy of the quantitation tests performed in this method are unknown. Concentrations are easier to determine in commercial products where asbestos was deliberately added because the amount is usually more than a few percent. An analyst's results can be "calibrated" against the known amounts added by the manufacturer. For geological samples, the degree of homogeneity affects the precision.
(iii) The performance of the method is analyst dependent. The analyst must choose carefully and not necessarily randomly the portions for analysis to assure that detection of asbestos occurs when it is present. For this reason, the analyst must have adequate training in sample preparation, and experience in the location and identification of asbestos in samples. This is usually accomplished through substantial on-the-job training as well as formal education in mineralogy and microscopy.
(e) Interferences
Any material which is long, thin, and small enough to be viewed under the microscope can be considered an interference for asbestos. There are literally hundreds of interferences in workplaces. The techniques described in this method are normally sufficient to eliminate the interferences. An analyst's success in eliminating the interferences depends on proper training.
Asbestos minerals belong to two mineral families: The serpentines and the amphiboles. In the serpentine family, the only common fibrous mineral is chrysotile. Occasionally, the mineral antigorite occurs in a fibril habit with morphology similar to the amphiboles. The amphibole minerals consist of a score of different minerals of which only five are regulated by federal standard: Amosite, crocidolite, anthophyllite asbestos, tremolite asbestos and actinolite asbestos. These are the only amphibole minerals that have been commercially exploited for their fibrous properties; however, the rest can and do occur occasionally in asbestiform habit.
In addition to the related mineral interferences, other minerals common in building material may present a problem for some microscopists: Gypsum, anhydrite, brucite, quartz fibers, talc fibers or ribbons, wollastonite, perlite, attapulgite, etc. Other fibrous materials commonly present in workplaces are: Fiberglass, mineral wool, ceramic wool, refractory ceramic fibers, kevlar, nomex, synthetic fibers, graphite or carbon fibers, cellulose (paper or wood) fibers, metal fibers, etc.
Matrix embedding material can sometimes be a negative interference. The analyst may not be able to easily extract the fibers from the matrix in order to use the method. Where possible, remove the matrix before the analysis, taking careful note of the loss of weight. Some common matrix materials are: Vinyl, rubber, tar, paint, plant fiber, cement, and epoxy. A further negative interference is that the asbestos fibers themselves may be either too small to be seen in Phase Contrast Microscopy (PCM) or of a very low fibrous quality, having the appearance of plant fibers. The analyst's ability to deal with these materials increases with experience.
(f) Uses and Occupational Exposure
Asbestos is ubiquitous in the environment. More than 40% of the land area of the United States is composed of minerals which may contain asbestos. Fortunately, the actual formation of great amounts of asbestos is relatively rare. Nonetheless, there are locations in which environmental exposure can be severe such as in the Serpentine Hills of California.
There are thousands of uses for asbestos in industry and the home. Asbestos abatement workers are the most current segment of the population to have occupational exposure to great amounts of asbestos. If the material is undisturbed, there is no exposure. Exposure occurs when the asbestos-containing material is abraded or otherwise disturbed during maintenance operations or some other activity. Approximately 95% of the asbestos in place in the United States is chrysotile.
Amosite and crocidolite make up nearly all the difference. Tremolite and anthophyllite make up a very small percentage. Tremolite is found in extremely small amounts in certain chrysotile deposits. Actinolite exposure is probably greatest from environmental sources, but has been identified in vermiculite containing, sprayed-on insulating materials which may have been certified as asbestos-free.
(g) Physical and Chemical Properties
The nominal chemical compositions for the asbestos minerals were given in subsection (1). Compared to cleavage fragments of the same minerals, asbestiform fibers possess a high tensile strength along the fiber axis. They are chemically inert, noncombustible, and heat resistant. Except for chrysotile, they are insoluble in Hydrochloric acid (HCl). Chrysotile is slightly soluble in HCl. Asbestos has high electrical resistance and good sound absorbing characteristics. It can be woven into cables, fabrics or other textiles, or matted into papers, felts, and mats.
(h) Toxicology (This Section is for Information Only and Should Not Be Taken as WISHA Policy)
Possible physiologic results of respiratory exposure to asbestos are mesothelioma of the pleura or peritoneum, interstitial fibrosis, asbestosis, pneumoconiosis, or respiratory cancer. The possible consequences of asbestos exposure are detailed in the NIOSH Criteria Document or in the WISHA Asbestos Standards, WAC 296-62-077.
(2) Sampling Procedure
(a) Equipment for Sampling
(i) Tube or cork borer sampling device
(ii) Knife
(iii) 20 mL scintillation vial or similar vial
(iv) Sealing encapsulant
(b) Safety Precautions
Asbestos is a known carcinogen. Take care when sampling. While in an asbestos-containing atmosphere, a properly selected and fit-tested respirator should be worn. Take samples in a manner to cause the least amount of dust. Follow these general guidelines:
(i) Do not make unnecessary dust.
(ii) Take only a small amount (1 to 2 g).
(iii) Tightly close the sample container.
(iv) Use encapsulant to seal the spot where the sample was taken, if necessary.
(c) Sampling procedure
Samples of any suspect material should be taken from an inconspicuous place. Where the material is to remain, seal the sampling wound with an encapsulant to eliminate the potential for exposure from the sample site. Microscopy requires only a few milligrams of material. The amount that will fill a 20 mL scintillation vial is more than adequate. Be sure to collect samples from all layers and phases of material. If possible, make separate samples of each different phase of the material. This will aid in determining the actual hazard. do not use envelopes, plastic or paper bags of any kind to collect samples. The use of plastic bags presents a contamination hazard to laboratory personnel and to other samples. When these containers are opened, a bellows effect blows fibers out of the container onto everything, including the person opening the container.
If a cork-borer type sampler is available, push the tube through the material all the way, so that all layers of material are sampled. Some samplers are intended to be disposable. These should be capped and sent to the laboratory. If a nondisposable cork borer is used, empty the contents into a scintillation vial and send to the laboratory. Vigorously and completely clean the cork borer between samples.
(d) Shipment
Samples packed in glass vials must not touch or they might break in shipment.
(i) Seal the samples with a sample seal over the end to guard against tampering and to identify the sample.
(ii) Package the bulk samples in separate packages from the air samples. They may cross-contaminate each other and will invalidate the results of the air samples.
(iii) Include identifying paperwork with the samples, but not in contact with the suspected asbestos.
(iv) To maintain sample accountability, ship the samples by certified mail, overnight express, or hand carry them to the laboratory.
(3) Analysis
The analysis of asbestos samples can be divided into two major parts: Sample preparation and microscopy. Because of the different asbestos uses that may be encountered by the analyst, each sample may need different preparation steps. The choices are outlined below. There are several different tests that are performed to identify the asbestos species and determine the percentage. They will be explained below.
(a) Safety
(i) Do not create unnecessary dust. Handle the samples in HEPA-filter equipped hoods. If samples are received in bags, envelopes or other inappropriate container, open them only in a hood having a face velocity at or greater than 100 fpm. Transfer a small amount to a scintillation vial and only handle the smaller amount.
(ii) Open samples in a hood, never in the open lab area.
(iii) Index of refraction oils can be toxic. Take care not to get this material on the skin. Wash immediately with soap and water if this happens.
(iv) Samples that have been heated in the muffle furnace or the drying oven may be hot. Handle them with tongs until they are cool enough to handle.
(v) Some of the solvents used, such as THF (tetrahydrofuran), are toxic and should only be handled in an appropriate fume hood and according to instructions given in the Safety Data Sheet (SDS).
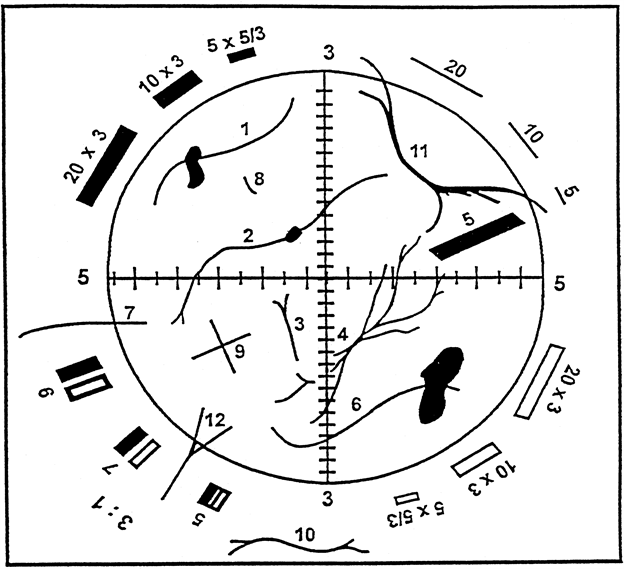 |
Figure 1: Walton-Beckett Graticule with some explanatory fibers. | ||
Counts for the Fibers in the Figure | ||
Structure No. | Count | Explanation |
1 to 6 | 1 | Single fibers all contained within the circle. |
7 | 1/2 | Fiber crosses circle once. |
8 | 0 | Fiber too short. |
9 | 2 | Two crossing fibers. |
10 | 0 | Fiber outside graticule. |
11 | 0 | Fiber crosses graticule twice. |
12 | 1/2 | Although split, fiber only crosses once. |
(b) Equipment
(i) Phase contrast microscope with 10x, 16x and 40x objectives, 10x wide-field eyepieces, G-22 Walton-Beckett graticule, Whipple disk, polarizer, analyzer and first order red or gypsum plate, 100 Watt illuminator, rotating position condenser with oversize phase rings, central stop dispersion objective, Kohler illumination and a rotating mechanical stage. (See Figure 1).
(ii) Stereo microscope with reflected light illumination, transmitted light illumination, polarizer, analyzer and first order red or gypsum plate, and rotating stage.
(iii) Negative pressure hood for the stereo microscope
(iv) Muffle furnace capable of 600 degrees C
(v) Drying oven capable of 50-150 degrees C
(vi) Aluminum specimen pans
(vii) Tongs for handling samples in the furnace
(viii) High dispersion index of refraction oils (Special for dispersion staining.)
n=1.550
n=1.585
n=1.590
n=1.605
n=1.620
n=1.670
n=1.680
n=1.690
(ix) A set of index of refraction oils from about n=1.350 to n=2.000 in n=0.005 increments. (Standard for Becke line analysis.)
(x) Glass slides with painted or frosted ends 1 x 3 inches 1mm thick, precleaned.
(xi) Cover Slips 22 x 22 mm, #1 1/2
(xii) Paper clips or dissection needles
(xiii) Hand grinder
(xiv) Scalpel with both #10 and #11 blades
(xv) 0.1 molar HCl
(xvi) Decalcifying solution (Baxter Scientific Products) Ethylenediaminetetraacetic Acid,
(xvii) Tetrasodium....0.7 g/l
Sodium Potassium Tartrate....8.0 mg/liter
Hydrochloric Acid....99.2 g/liter
Sodium Tartrate....0.14 g/liter
Tetrahydrofuran (THF)
(xviii) Hotplate capable of 60 degrees C
(xix) Balance
(xx) Hacksaw blade
(xxi) Ruby mortar and pestle
(c) Sample Pre-Preparation
Sample preparation begins with pre-preparation which may include chemical reduction of the matrix, heating the sample to dryness or heating in the muffle furnace. The end result is a sample which has been reduced to a powder that is sufficiently fine to fit under the cover slip. Analyze different phases of samples separately, e.g., tile and the tile mastic should be analyzed separately as the mastic may contain asbestos while the tile may not.
(i) Wet Samples
Samples with a high water content will not give the proper dispersion colors and must be dried prior to sample mounting. Remove the lid of the scintillation vial, place the bottle in the drying oven and heat at 100 degrees C to dryness (usually about 2 h). Samples which are not submitted to the lab in glass must be removed and placed in glass vials or aluminum weighing pans before placing them in the drying oven.
(ii) Samples With Organic Interference-Muffle Furnace
These may include samples with tar as a matrix, vinyl asbestos tile, or any other organic that can be reduced by heating. Remove the sample from the vial and weigh in a balance to determine the weight of the submitted portion. Place the sample in a muffle furnace at 500 degrees C for 1 to 2 h or until all obvious organic material has been removed. Retrieve, cool and weigh again to determine the weight loss on ignition. This is necessary to determine the asbestos content of the submitted sample, because the analyst will be looking at a reduced sample.
Notes: Heating above 600 degrees C will cause the sample to undergo a structural change which, given sufficient time, will convert the chrysotile to forsterite. Heating even at lower temperatures for 1 to 2 h may have a measurable effect on the optical properties of the minerals. If the analyst is unsure of what to expect, a sample of standard asbestos should be heated to the same temperature for the same length of time so that it can be examined for the proper interpretation.
(iii) Samples With Organic Interference-THF
Vinyl asbestos tile is the most common material treated with this solvent, although, substances containing tar will sometimes yield to this treatment. Select a portion of the material and then grind it up if possible. Weigh the sample and place it in a test tube. Add sufficient THF to dissolve the organic matrix. This is usually about 4 to 5 mL. Remember, THF is highly flammable. Filter the remaining material through a tared silver membrane, dry and weigh to determine how much is left after the solvent extraction. Further process the sample to remove carbonate or mount directly.
(iv) Samples With Carbonate Interference
Carbonate material is often found on fibers and sometimes must be removed in order to perform dispersion microscopy. Weigh out a portion of the material and place it in a test tube. Add a sufficient amount of 0.1 M HCl or decalcifying solution in the tube to react all the carbonate as evidenced by gas formation; i.e., when the gas bubbles stop, add a little more solution. If no more gas forms, the reaction is complete. Filter the material out through a tared silver membrane, dry and weigh to determine the weight lost.
(d) Sample Preparation
Samples must be prepared so that accurate determination can be made of the asbestos type and amount present. The following steps are carried out in the low-flow hood (a low-flow hood has less than 50 fpm flow):
(i) If the sample has large lumps, is hard, or cannot be made to lie under a cover slip, the grain size must be reduced. Place a small amount between two slides and grind the material between them or grind a small amount in a clean mortar and pestle. The choice of whether to use an alumina, ruby, or diamond mortar depends on the hardness of the material. Impact damage can alter the asbestos mineral if too much mechanical shock occurs. (Freezer mills can completely destroy the observable crystallinity of asbestos and should not be used). For some samples, a portion of material can be shaved off with a scalpel, ground off with a hand grinder or hacksaw blade.
The preparation tools should either be disposable or cleaned thoroughly. Use vigorous scrubbing to loosen the fibers during the washing. Rinse the implements with copious amounts of water and air-dry in a dust-free environment.
(ii) If the sample is powder or has been reduced as in (i) above, it is ready to mount. Place a glass slide on a piece of optical tissue and write the identification on the painted or frosted end. Place two drops of index of refraction medium n=1.550 on the slide. (The medium n=1.550 is chosen because it is the matching index for chrysotile.) Dip the end of a clean paper-clip or dissecting needle into the droplet of refraction medium on the slide to moisten it. Then dip the probe into the powder sample. Transfer what sticks on the probe to the slide. The material on the end of the probe should have a diameter of about 3 mm for a good mount. If the material is very fine, less sample may be appropriate. For nonpowder samples such as fiber mats, forceps should be used to transfer a small amount of material to the slide. Stir the material in the medium on the slide, spreading it out and making the preparation as uniform as possible. Place a cover-slip on the preparation by gently lowering onto the slide and allowing it to fall "trapdoor" fashion on the preparation to push out any bubbles. Press gently on the cover slip to even out the distribution of particulate on the slide. If there is insufficient mounting oil on the slide, one or two drops may be placed near the edge of the coverslip on the slide. Capillary action will draw the necessary amount of liquid into the preparation. Remove excess oil with the point of a laboratory wiper.
Treat at least two different areas of each phase in this fashion. Choose representative areas of the sample. It may be useful to select particular areas or fibers for analysis. This is useful to identify asbestos in severely inhomogeneous samples.
When it is determined that amphiboles may be present, repeat the above process using the appropriate high-dispersion oils until an identification is made or all six asbestos minerals have been ruled out. Note that percent determination must be done in the index medium 1.550 because amphiboles tend to disappear in their matching mediums.
(e) Analytical procedure
Note: This method presumes some knowledge of mineralogy and optical petrography.
The analysis consists of three parts: The determination of whether there is asbestos present, what type is present and the determination of how much is present. The general flow of the analysis is:
(i) Gross examination.
(ii) Examination under polarized light on the stereo microscope.
(iii) Examination by phase-polar illumination on the compound phase microscope.
(iv) Determination of species by dispersion stain. Examination by Becke line analysis may also be used; however, this is usually more cumbersome for asbestos determination.
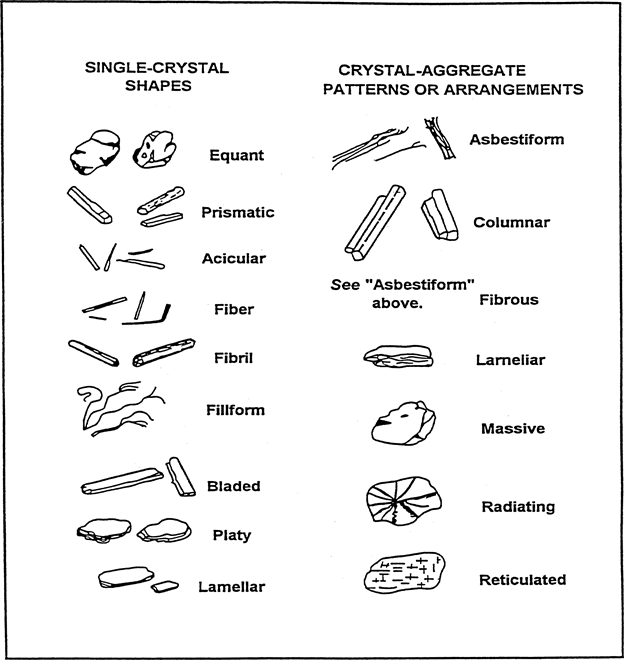 |
Figure 1. Particle definitions showing mineral growth habits. From the U.S. Bureau of Mines. |
(v) Difficult samples may need to be analyzed by SEM or TEM, or the results from those techniques combined with light microscopy for a definitive identification. Identification of a particle as asbestos requires that it be asbestiform. Description of particles should follow the suggestion of Campbell. (Figure 2)
For the purpose of regulation, the mineral must be one of the six minerals covered and must be in the asbestos growth habit. Large specimen samples of asbestos generally have the gross appearance of wood. Fibers are easily parted from it. Asbestos fibers are very long compared with their widths. The fibers have a very high tensile strength as demonstrated by bending without breaking. Asbestos fibers exist in bundles that are easily parted, show longitudinal fine structure and may be tufted at the ends showing "bundle of sticks" morphology. In the microscope some of these properties may not be observable. Amphiboles do not always show striations along their length even when they are asbestos. Neither will they always show tufting. They generally do not show a curved nature except for very long fibers. Asbestos and asbestiform minerals are usually characterized in groups by extremely high aspect ratios (greater than 100:1). While aspect ratio analysis is useful for characterizing populations of fibers, it cannot be used to identify individual fibers of intermediate to short aspect ratio. Observation of many fibers is often necessary to determine whether a sample consists of "cleavage fragments" or of asbestos fibers.
Most cleavage fragments of the asbestos minerals are easily distinguishable from true asbestos fibers. This is because true cleavage fragments usually have larger diameters than 1 micron. Internal structure of particles larger than this usually shows them to have no internal fibrillar structure. In addition, cleavage fragments of the monoclinic amphiboles show inclined extinction under crossed polars with no compensator. Asbestos fibers usually show extinction at zero degrees or ambiguous extinction if any at all. Morphologically, the larger cleavage fragments are obvious by their blunt or stepped ends showing prismatic habit. Also, they tend to be acicular rather than filiform.
Where the particles are less than 1 micron in diameter and have an aspect ratio greater than or equal to 3:1, it is recommended that the sample be analyzed by SEM or TEM if there is any question whether the fibers are cleavage fragments or asbestiform particles.
Care must be taken when analyzing by electron microscopy because the interferences are different from those in light microscopy and may structurally be very similar to asbestos. The classic interference is between anthophyllite and biopyribole or intermediate fiber. Use the same morphological clues for electron microscopy as are used for light microscopy, e.g. fibril splitting, internal longitudinal striation, fraying, curvature, etc.
(vi) Gross examination:
Examine the sample, preferably in the glass vial. Determine the presence of any obvious fibrous component. Estimate a percentage based on previous experience and current observation. Determine whether any pre-preparation is necessary. Determine the number of phases present. This step may be carried out or augmented by observation at 6x to 40x under a stereo microscope.
(vii) After performing any necessary pre-preparation, prepare slides of each phase as described above. Two preparations of the same phase in the same index medium can be made side-by-side on the same glass for convenience. Examine with the polarizing stereo microscope. Estimate the percentage of asbestos based on the amount of birefringent fiber present.
(viii) Examine the slides on the phase-polar microscopes at magnifications of 160x and 400x. Note the morphology of the fibers. Long, thin, very straight fibers with little curvature are indicative of fibers from the amphibole family. Curved, wavy fibers are usually indicative of chrysotile. Estimate the percentage of asbestos on the phase-polar microscope under conditions of crossed polars and a gypsum plate. Fibers smaller than 1.0 microns in thickness must be identified by inference to the presence of larger, identifiable fibers and morphology. If no larger fibers are visible, electron microscopy should be performed. At this point, only a tentative identification can be made. Full identification must be made with dispersion microscopy. Details of the tests are included in the appendices.
(ix) Once fibers have been determined to be present, they must be identified. Adjust the microscope for dispersion mode and observe the fibers. The microscope has a rotating stage, one polarizing element, and a system for generating dark-field dispersion microscopy (see subsection (4)(f) of this appendix). Align a fiber with its length parallel to the polarizer and note the color of the Becke lines. Rotate the stage to bring the fiber length perpendicular to the polarizer and note the color. Repeat this process for every fiber or fiber bundle examined. The colors must be consistent with the colors generated by standard asbestos reference materials for a positive identification. In n=1.550, amphiboles will generally show a yellow to straw-yellow color indicating that the fiber indices of refraction are higher than the liquid. If long, thin fibers are noted and the colors are yellow, prepare further slides as above in the suggested matching liquids listed below:
Type of asbestos | Index of refraction |
Chrysotile | n=1.550. |
Amosite | n=1.670 or 1.680. |
Crocidolite | n=1.690. |
Anthophyllite | n=1.605 and 1.620. |
Tremolite | n=1.605 and 1.620. |
Actinolite | n=1.620. |
Where more than one liquid is suggested, the first is preferred; however, in some cases this liquid will not give good dispersion color. Take care to avoid interferences in the other liquid; e.g., wollastonite in n=1.620 will give the same colors as tremolite. In n=1.605 wollastonite will appear yellow in all directions. Wollastonite may be determined under crossed polars as it will change from blue to yellow as it is rotated along its fiber axis by tapping on the cover slip. Asbestos minerals will not change in this way.
Determination of the angle of extinction may, when present, aid in the determination of anthophyllite from tremolite. True asbestos fibers usually have 0 degree extinction or ambiguous extinction, while cleavage fragments have more definite extinction.
Continue analysis until both preparations have been examined and all present species of asbestos are identified. If there are no fibers present, or there is less than 0.1% present, end the analysis with the minimum number of slides (2).
(x) Some fibers have a coating on them which makes dispersion microscopy very difficult or impossible. Becke line analysis or electron microscopy may be performed in those cases. Determine the percentage by light microscopy. TEM analysis tends to overestimate the actual percentage present.
(xi) Percentage determination is an estimate of occluded area, tempered by gross observation. Gross observation information is used to make sure that the high magnification microscopy does not greatly over- or under-estimate the amount of fiber present. This part of the analysis requires a great deal of experience. Satisfactory models for asbestos content analysis have not yet been developed, although some models based on metallurgical grain-size determination have found some utility. Estimation is more easily handled in situations where the grain sizes visible at about 160x are about the same and the sample is relatively homogeneous.
View all of the area under the cover slip to make the percentage determination. View the fields while moving the stage, paying attention to the clumps of material. These are not usually the best areas to perform dispersion microscopy because of the interference from other materials. But, they are the areas most likely to represent the accurate percentage in the sample. Small amounts of asbestos require slower scanning and more frequent analysis of individual fields.
Report the area occluded by asbestos as the concentration. This estimate does not generally take into consideration the difference in density of the different species present in the sample. For most samples this is adequate. Simulation studies with similar materials must be carried out to apply microvisual estimation for that purpose and is beyond the scope of this procedure.
(xii) Where successive concentrations have been made by chemical or physical means, the amount reported is the percentage of the material in the "as submitted" or original state. The percentage determined by microscopy is multiplied by the fractions remaining after pre-preparation steps to give the percentage in the original sample. For example:
Step 1. 60% remains after heating at 550 degrees C for 1 h.
Step 2. 30% of the residue of step 1 remains after dissolution of carbonate in 0.1 m HCl.
Step 3. Microvisual estimation determines that 5% of the sample is chrysotile asbestos.
The reported result is:
R=(Microvisual result in percent)x(Fraction remaining after step 2)x(Fraction remaining of original sample after step 1)
R=(5)x(.30)x(.60)=0.9%
(xiii) Report the percent and type of asbestos present. For samples where asbestos was identified, but is less than 1.0%, report "Asbestos present, less than 1.0%." There must have been at least two observed fibers or fiber bundles in the two preparations to be reported as present. For samples where asbestos was not seen, report as "None Detected."
(4) Auxiliary Information
Because of the subjective nature of asbestos analysis, certain concepts and procedures need to be discussed in more depth. This information will help the analyst understand why some of the procedures are carried out the way they are.
(a) Light
Light is electromagnetic energy. It travels from its source in packets called quanta. It is instructive to consider light as a plane wave. The light has a direction of travel. Perpendicular to this and mutually perpendicular to each other, are two vector components. One is the magnetic vector and the other is the electric vector. We shall only be concerned with the electric vector. In this description, the interaction of the vector and the mineral will describe all the observable phenomena. From a light source such a microscope illuminator, light travels in all different direction from the filament.
In any given direction away from the filament, the electric vector is perpendicular to the direction of travel of a light ray. While perpendicular, its orientation is random about the travel axis. If the electric vectors from all the light rays were lined up by passing the light through a filter that would only let light rays with electric vectors oriented in one direction pass, the light would then be polarized.
Polarized light interacts with matter in the direction of the electric vector. This is the polarization direction. Using this property it is possible to use polarized light to probe different materials and identify them by how they interact with light. The speed of light in a vacuum is a constant at about 2.99×108m/s. When light travels in different materials such as air, water, minerals or oil, it does not travel at this speed. It travels slower. This slowing is a function of both the material through which the light is traveling and the wavelength or frequency of the light. In general, the more dense the material, the slower the light travels. Also, generally, the higher the frequency, the slower the light will travel. The ratio of the speed of light in a vacuum to that in a material is called the index of refraction (n). It is usually measured at 589 nm (the sodium D line). If white light (light containing all the visible wavelengths) travels through a material, rays of longer wavelengths will travel faster than those of shorter wavelengths, this separation is called dispersion. Dispersion is used as an identifier of materials as described in Section (4)(f).
(b) Material Properties
Materials are either amorphous or crystalline. The difference between these two descriptions depends on the positions of the atoms in them. The atoms in amorphous materials are randomly arranged with no long range order. An example of an amorphous material is glass. The atoms in crystalline materials, on the other hand, are in regular arrays and have long range order. Most of the atoms can be found in highly predictable locations. Examples of crystalline material are salt, gold, and the asbestos minerals.
It is beyond the scope of this method to describe the different types of crystalline materials that can be found, or the full description of the classes into which they can fall. However, some general crystallography is provided below to give a foundation to the procedures described.
With the exception of anthophyllite, all the asbestos minerals belong to the monoclinic crystal type. The unit cell is the basic repeating unit of the crystal and for monoclinic crystals can be described as having three unequal sides, two 90 degrees angles and one angle not equal to 90 degrees. The orthorhombic group, of which anthophyllite is a member has three unequal sides and three 90 degrees angles. The unequal sides are a consequence of the complexity of fitting the different atoms into the unit cell. Although the atoms are in a regular array, that array is not symmetrical in all directions. There is long range order in the three major directions of the crystal. However, the order is different in each of the three directions. This has the effect that the index of refraction is different in each of the three directions. Using polarized light, we can investigate the index of refraction in each of the directions and identify the mineral or material under investigation. The indices alpha, beta, and gamma are used to identify the lowest, middle, and highest index of refraction respectively. The x direction, associated with alpha is called the fast axis. Conversely, the z direction is associated with gamma and is the slow direction. Crocidolite has alpha along the fiber length making it "length-fast." The remainder of the asbestos minerals have the gamma axis along the fiber length. They are called "length-slow." This orientation to fiber length is used to aid in the identification of asbestos.
(c) Polarized Light Technique
Polarized light microscopy as described in this section uses the phase-polar microscope described in Section (3)(b). A phase contrast microscope is fitted with two polarizing elements, one below and one above the sample. The polarizers have their polarization directions at right angles to each other. Depending on the tests performed, there may be a compensator between these two polarizing elements. Light emerging from a polarizing element has its electric vector pointing in the polarization direction of the element. The light will not be subsequently transmitted through a second element set at a right angle to the first element. Unless the light is altered as it passes from one element to the other, there is no transmission of light.
(d) Angle of Extinction
Crystals which have different crystal regularity in two or three main directions are said to be anisotropic. They have a different index of refraction in each of the main directions. When such a crystal is inserted between the crossed polars, the field of view is no longer dark but shows the crystal in color. The color depends on the properties of the crystal. The light acts as if it travels through the crystal along the optical axes. If a crystal optical axis were lined up along one of the polarizing directions (either the polarizer or the analyzer) the light would appear to travel only in that direction, and it would blink out or go dark. The difference in degrees between the fiber direction and the angle at which it blinks out is called the angle of extinction. When this angle can be measured, it is useful in identifying the mineral. The procedure for measuring the angle of extinction is to first identify the polarization direction in the microscope. A commercial alignment slide can be used to establish the polarization directions or use anthophyllite or another suitable mineral. This mineral has a zero degree angle of extinction and will go dark to extinction as it aligns with the polarization directions. When a fiber of anthophyllite has gone to extinction, align the eyepiece reticle or graticule with the fiber so that there is a visual cue as to the direction of polarization in the field of view. Tape or otherwise secure the eyepiece in this position so it will not shift.
After the polarization direction has been identified in the field of view, move the particle of interest to the center of the field of view and align it with the polarization direction. For fibers, align the fiber along this direction. Note the angular reading of the rotating stage. Looking at the particle, rotate the stage until the fiber goes dark or "blinks out." Again note the reading of the stage. The difference in the first reading and the second is an angle of extinction.
The angle measured may vary as the orientation of the fiber changes about its long axis. Tables of mineralogical data usually report the maximum angle of extinction. Asbestos forming minerals, when they exhibit an angle of extinction, usually do show an angle of extinction close to the reported maximum, or as appropriate depending on the substitution chemistry.
(e) Crossed Polars With Compensator
When the optical axes of a crystal are not lined up along one of the polarizing directions (either the polarizer or the analyzer) part of the light travels along one axis and part travels along the other visible axis. This is characteristic of birefringent materials.
The color depends on the difference of the two visible indices of refraction and the thickness of the crystal. The maximum difference available is the difference between the alpha and the gamma axises. This maximum difference is usually tabulated as the birefringence of the crystal.
For this test, align the fiber at 45 degrees to the polarization directions in order to maximize the contribution to each of the optical axes. The colors seen are called retardation colors. They arise from the recombination of light which has traveled through the two separate directions of the crystal. One of the rays is retarded behind the other since the light in that direction travels slower. On recombination, some of the colors which make up white light are enhanced by constructive interference and some are suppressed by destructive interference. The result is a color dependent on the difference between the indices and the thickness of the crystal. The proper colors, thicknesses, and retardations are shown on a Michel-Levy chart. The three items, retardation, thickness and birefringence are related by the following relationship: Lambda
R = t (n γ— α) | |
R= retardation, t= crystal thickness in micron, and alpha, gamma= indices of refraction. | |
Examination of the equation for asbestos minerals reveals that the visible colors for almost all common asbestos minerals and fiber sizes are shades of gray and black. The eye is relatively poor at discriminating different shades of gray. It is very good at discriminating different colors. In order to compensate for the low retardation, a compensator is added to the light train between the polarization elements. The compensator used for this test is a gypsum plate of known thickness and birefringence. Such a compensator when oriented at 45 degrees to the polarizer direction, provides a retardation of 530 nm of the 530 nm wavelength color. This enhances the red color and gives the background a characteristic red to red-magenta color. If this "full-wave" compensator is in place when the asbestos preparation is inserted into the light train, the colors seen on the fibers are quite different. Gypsum, like asbestos has a fast axis and a slow axis. When a fiber is aligned with its fast axis in the same direction as the fast axis of the gypsum plate, the ray vibrating in the slow direction is retarded by both the asbestos and the gypsum. This results in a higher retardation than would be present for either of the two minerals. The color seen is a second order blue. When the fiber is rotated 90 degrees using the rotating stage, the slow direction of the fiber is now aligned with the fast direction of the gypsum and the fast direction of the fiber is aligned with the slow direction of the gypsum. Thus, one ray vibrates faster in the fast direction of the gypsum, and slower in the slow direction of the fiber; the other ray will vibrate slower in the slow direction of the gypsum and faster in the fast direction of the fiber. In this case, the effect is subtractive and the color seen is a first order yellow. As long as the fiber thickness does not add appreciably to the color, the same basic colors will be seen for all asbestos types except crocidolite. In crocidolite the colors will be weaker, may be in the opposite directions, and will be altered by the blue absorption color natural to crocidolite. Hundreds of other materials will give the same colors as asbestos, and therefore, this test is not definitive for asbestos. The test is useful in discriminating against fiberglass or other amorphous fibers such as some synthetic fibers. Certain synthetic fibers will show retardation colors different than asbestos; however, there are some forms of polyethylene and aramid which will show morphology and retardation colors similar to asbestos minerals. This test must be supplemented with a positive identification test when birefringent fibers are present which can not be excluded by morphology. This test is relatively ineffective for use on fibers less than 1 micron in diameter. For positive confirmation TEM or SEM should be used if no larger bundles or fibers are visible.
(f) Dispersion Staining
Dispersion microscopy or dispersion staining is the method of choice for the identification of asbestos in bulk materials. Becke line analysis is used by some laboratories and yields the same results as does dispersion staining for asbestos and can be used in lieu of dispersion staining. Dispersion staining is performed on the same platform as the phase-polar analysis with the analyzer and compensator removed. One polarizing element remains to define the direction of the light so that the different indices of refraction of the fibers may be separately determined. Dispersion microscopy is a dark-field technique when used for asbestos. Particles are imaged with scattered light. Light which is unscattered is blocked from reaching the eye either by the back field image mask in a McCrone objective or a back field image mask in the phase condenser. The most convenient method is to use the rotating phase condenser to move an oversized phase ring into place. The ideal size for this ring is for the central disk to be just larger than the objective entry aperture as viewed in the back focal plane. The larger the disk, the less scattered light reaches the eye. This will have the effect of diminishing the intensity of dispersion color and will shift the actual color seen. The colors seen vary even on microscopes from the same manufacturer. This is due to the different bands of wavelength exclusion by different mask sizes. The mask may either reside in the condenser or in the objective back focal plane. It is imperative that the analyst determine by experimentation with asbestos standards what the appropriate colors should be for each asbestos type. The colors depend also on the temperature of the preparation and the exact chemistry of the asbestos. Therefore, some slight differences from the standards should be allowed. This is not a serious problem for commercial asbestos uses. This technique is used for identification of the indices of refraction for fibers by recognition of color. There is no direct numerical readout of the index of refraction. Correlation of color to actual index of refraction is possible by referral to published conversion tables. This is not necessary for the analysis of asbestos. Recognition of appropriate colors along with the proper morphology are deemed sufficient to identify the commercial asbestos minerals. Other techniques including SEM, TEM, and XRD may be required to provide additional information in order to identify other types of asbestos.
Make a preparation in the suspected matching high dispersion oil, e.g., n=1.550 for chrysotile. Perform the preliminary tests to determine whether the fibers are birefringent or not. Take note of the morphological character. Wavy fibers are indicative of chrysotile while long, straight, thin, frayed fibers are indicative of amphibole asbestos. This can aid in the selection of the appropriate matching oil. The microscope is set up and the polarization direction is noted as in Section (4)(d). Align a fiber with the polarization direction. Note the color. This is the color parallel to the polarizer. Then rotate the fiber rotating the stage 90 degrees so that the polarization direction is across the fiber. This is the perpendicular position. Again note the color. Both colors must be consistent with standard asbestos minerals in the correct direction for a positive identification of asbestos. If only one of the colors is correct while the other is not, the identification is not positive. If the colors in both directions are bluish-white, the analyst has chosen a matching index oil which is higher than the correct matching oil, e.g. the analyst has used n= 1.620 where chrysotile is present. The next lower oil (Section (3)(e)) should be used to prepare another specimen. If the color in both directions is yellow-white to straw-yellow-white, this indicates that the index of the oil is lower than the index of the fiber, e.g. the preparation is in n= 1.550 while anthophyllite is present. Select the next higher oil (Section (3)(e)) and prepare another slide. Continue in this fashion until a positive identification of all asbestos species present has been made or all possible asbestos species have been ruled out by negative results in this test. Certain plant fibers can have similar dispersion colors as asbestos. Take care to note and evaluate the morphology of the fibers or remove the plant fibers in prepreparation. Coating material on the fibers such as carbonate or vinyl may destroy the dispersion color. Usually, there will be some outcropping of fiber which will show the colors sufficient for identification. When this is not the case, treat the sample as described in Section (3)(c) and then perform dispersion staining. Some samples will yield to Becke line analysis if they are coated or electron microscopy can be used for identification.
(5) References
Crane, D.T., Asbestos in Air, OSHA method ID160, Revised November 1992.
Ford, W.E., Dana's Textbook of Mineralogy; Fourth Ed.; John Wiley and Son, New York, 1950, p. vii.
Selikoff,.I.J., Lee, D.H.K., Asbestos and Disease, Academic Press, New York, 1978, pp. 3, 20.
Women Inspectors of Factories. Annual Report for 1898, H.M. Statistical Office, London, p. 170 (1898).
Selikoff,.I.J., Lee, D.H.K., Asbestos and Disease, Academic Press, New York, 1978, pp. 26, 30.
Campbell, W.J., et al, Selected Silicate Minerals and Their Asbestiform Varieties, United States Department of the Interior, Bureau of Mines, Information Circular 8751, 1977.
Asbestos, Code of Federal Regulations, 29 C.F.R. 1910.1001 and 29 C.F.R. 1926.58.
National Emission Standards for Hazardous Air Pollutants; Asbestos NESHAP Revision, Federal Register, Vol. 55, No. 224, 20 November 1990, p. 48410.
Ross, M. The Asbestos Minerals: Definitions, Description, Modes of Formation, Physical and Chemical Properties and Health Risk to the Mining Community, Nation Bureau of Standards Special Publication, Washington, D.C., 1977.
Lilis, R., Fibrous Zeolites and Endemic Mesothelioma in Cappadocia, Turkey, J. Occ Medicine, 1981, 23, (8), 548-550.
Occupational Exposure to Asbestos-1972, U.S. Department of Health Education and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, HSM-72-10267.
Campbell, W.J., et al, Relationship of Mineral Habit to Size Characteristics for Tremolite Fragments and Fibers, United States Department of the Interior, Bureau of Mines, Information Circular 8367, 1979.
Mefford, D., DCM Laboratory, Denver, private communication, July 1987.
Deer, W.A., Howie, R.A., Zussman, J., Rock Forming Minerals, Longman, Thetford, UK, 1974.
Kerr, P.F., Optical Mineralogy; Third Ed. McGraw-Hill, New York, 1959.
Veblen, D.R. (Ed.), Amphiboles and Other Hydrous Pyriboles-Mineralogy, Reviews in Mineralogy, Vol. 9A, Michigan, 1982, pp 1-102.
Dixon, W.C., Applications of Optical Microscopy in the Analysis of Asbestos and Quartz, ACS Symposium Series, No. 120, Analytical Techniques in Occupational Health Chemistry, 1979.
Polarized Light Microscopy, McCrone Research Institute, Chicago, 1976.
Asbestos Identification, McCrone Research Institute, G &G printers, Chicago, 1987.
McCrone, W.C., Calculation of Refractive Indices from Dispersion Staining Data, The Microscope, No. 37, Chicago, 1989.
Levadie, B. (Ed.), Asbestos and Other Health Related Silicates, ASTM Technical Publication 834, ASTM, Philadelphia 1982.
Steel, E. and Wylie, A., Riordan, P.H. (Ed.), Mineralogical Characteristics of Asbestos, Geology of Asbestos Deposits, pp. 93-101, SME-AIME, 1981.
Zussman, J., The Mineralogy of Asbestos, Asbestos: Properties, Applications and Hazards, pp. 45-67 Wiley, 1979.